Exercise performance and peripheral vascular insufficiency improve with AMPK activation in high-fat diet-fed mice
- PMID: 24561866
- PMCID: PMC3989755
- DOI: 10.1152/ajpheart.00839.2013
Exercise performance and peripheral vascular insufficiency improve with AMPK activation in high-fat diet-fed mice
Abstract
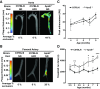
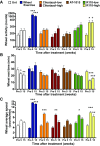
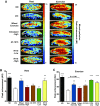
Intermittent claudication is a form of exercise intolerance characterized by muscle pain during walking in patients with peripheral artery disease (PAD). Endothelial cell and muscle dysfunction are thought to be important contributors to the etiology of this disease, but a lack of preclinical models that incorporate these elements and measure exercise performance as a primary end point has slowed progress in finding new treatment options for these patients. We sought to develop an animal model of peripheral vascular insufficiency in which microvascular dysfunction and exercise intolerance were defining features. We further set out to determine if pharmacological activation of 5'-AMP-activated protein kinase (AMPK) might counteract any of these functional deficits. Mice aged on a high-fat diet demonstrate many functional and molecular characteristics of PAD, including the sequential development of peripheral vascular insufficiency, increased muscle fatigability, and progressive exercise intolerance. These changes occur gradually and are associated with alterations in nitric oxide bioavailability. Treatment of animals with an AMPK activator, R118, increased voluntary wheel running activity, decreased muscle fatigability, and prevented the progressive decrease in treadmill exercise capacity. These functional performance benefits were accompanied by improved mitochondrial function, the normalization of perfusion in exercising muscle, increased nitric oxide bioavailability, and decreased circulating levels of the endogenous endothelial nitric oxide synthase inhibitor asymmetric dimethylarginine. These data suggest that aged, obese mice represent a novel model for studying exercise intolerance associated with peripheral vascular insufficiency, and pharmacological activation of AMPK may be a suitable treatment for intermittent claudication associated with PAD.
Keywords: 5′-AMP-activated protein kinase; exercise; intermittent claudication; nitric oxide; obesity.
Figures









References
-
- Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab 295: E1323–E1332, 2008 - PubMed
-
- Bauer TA, Regensteiner JG, Brass EP, Hiatt WR. Oxygen uptake kinetics during exercise are slowed in patients with peripheral arterial disease. J Appl Physiol 87: 809–816, 1999 - PubMed
-
- Bhat HK, Hiatt WR, Hoppel CL, Brass EP. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation 99: 807–812, 1999 - PubMed
-
- Bhattacharya I, Mundy AL, Widmer CC, Kretz M, Barton M. Regional heterogeneity of functional changes in conduit arteries after high-fat diet. Obesity 16: 743–748, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

