Opioids induce dissociable forms of long-term depression of excitatory inputs to the dorsal striatum
- PMID: 24561996
- PMCID: PMC4163916
- DOI: 10.1038/nn.3652
Opioids induce dissociable forms of long-term depression of excitatory inputs to the dorsal striatum
Abstract
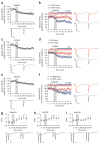
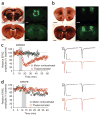
As prescription opioid analgesic abuse rates rise, so does the need to understand the long-term effects of opioid exposure on brain function. The dorsal striatum is an important site for drug-induced neuronal plasticity. We found that exogenously applied and endogenously released opioids induced long-term depression (OP-LTD) of excitatory inputs to the dorsal striatum in mice and rats. Mu and delta OP-LTD, although both being presynaptically expressed, were dissociable in that they summated, differentially occluded endocannabinoid-LTD and inhibited different striatal inputs. Kappa OP-LTD showed a unique subregional expression in striatum. A single in vivo exposure to the opioid analgesic oxycodone disrupted mu OP-LTD and endocannabinoid-LTD, but not delta or kappa OP-LTD. These data reveal previously unknown opioid-mediated forms of long-term striatal plasticity that are differentially affected by opioid analgesic exposure and are likely important mediators of striatum-dependent learning and behavior.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Cicero TJ, Inciardi JA, Muñoz A. Trends in abuse of oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain. 2005;6:662–672. - PubMed
-
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. - PubMed
-
- Ordóñez Gallego A, González Barón M, Espinosa Arranz E. Oxycodone: a pharmacological and clinical review. Clin Transl Oncol. 2007;9:298–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

