Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells
- PMID: 24562309
- PMCID: PMC4005806
- DOI: 10.1038/ni.2830
Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells
Abstract
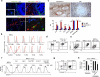
Innate lymphoid cells (ILCs) regulate stromal cells, epithelial cells and cells of the immune system, but their effect on B cells remains unclear. Here we identified RORγt(+) ILCs near the marginal zone (MZ), a splenic compartment that contains innate-like B cells highly responsive to circulating T cell-independent (TI) antigens. Splenic ILCs established bidirectional crosstalk with MAdCAM-1(+) marginal reticular cells by providing tumor-necrosis factor (TNF) and lymphotoxin, and they stimulated MZ B cells via B cell-activation factor (BAFF), the ligand of the costimulatory receptor CD40 (CD40L) and the Notch ligand Delta-like 1 (DLL1). Splenic ILCs further helped MZ B cells and their plasma-cell progeny by coopting neutrophils through release of the cytokine GM-CSF. Consequently, depletion of ILCs impaired both pre- and post-immune TI antibody responses. Thus, ILCs integrate stromal and myeloid signals to orchestrate innate-like antibody production at the interface between the immune system and circulatory system.
Figures





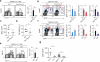

Comment in
-
Innate lymphoid cells: support for indie B cells.Nat Rev Immunol. 2014 Apr;14(4):213. doi: 10.1038/nri3644. Epub 2014 Mar 14. Nat Rev Immunol. 2014. PMID: 24625843 No abstract available.
-
ILCs in the zone.Nat Immunol. 2014 Apr;15(4):313-4. doi: 10.1038/ni.2854. Nat Immunol. 2014. PMID: 24646589 No abstract available.
Similar articles
-
Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses.Immunity. 2002 Sep;17(3):341-52. doi: 10.1016/s1074-7613(02)00389-8. Immunity. 2002. PMID: 12354386
-
A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells.Nature. 2013 Feb 14;494(7436):261-5. doi: 10.1038/nature11813. Epub 2013 Jan 16. Nature. 2013. PMID: 23334414
-
Ageing adversely affects the migration and function of marginal zone B cells.Immunology. 2017 Jul;151(3):349-362. doi: 10.1111/imm.12737. Epub 2017 May 4. Immunology. 2017. PMID: 28369800 Free PMC article.
-
Re-thinking the functions of IgA(+) plasma cells.Gut Microbes. 2014;5(5):652-62. doi: 10.4161/19490976.2014.969977. Gut Microbes. 2014. PMID: 25483334 Free PMC article. Review.
-
Aryl hydrocarbon receptor promotes RORγt⁺ group 3 ILCs and controls intestinal immunity and inflammation.Semin Immunopathol. 2013 Nov;35(6):657-70. doi: 10.1007/s00281-013-0393-5. Epub 2013 Aug 23. Semin Immunopathol. 2013. PMID: 23975386 Free PMC article. Review.
Cited by
-
Group 3 innate lymphoid cells require BATF to regulate gut homeostasis in mice.J Exp Med. 2022 Nov 7;219(11):e20211861. doi: 10.1084/jem.20211861. Epub 2022 Sep 1. J Exp Med. 2022. PMID: 36048018 Free PMC article.
-
Cutting Edge: Neutrophil Complement Receptor Signaling Is Required for BAFF-Dependent Humoral Responses in Mice.J Immunol. 2023 Jan 1;210(1):19-23. doi: 10.4049/jimmunol.2200410. J Immunol. 2023. PMID: 36454023 Free PMC article.
-
mTORC1 activation in B cells confers impairment of marginal zone microarchitecture by exaggerating cathepsin activity.Immunology. 2018 Dec;155(4):505-518. doi: 10.1111/imm.12996. Epub 2018 Sep 16. Immunology. 2018. PMID: 30144045 Free PMC article.
-
Antigen-presenting ILC3 regulate T cell-dependent IgA responses to colonic mucosal bacteria.J Exp Med. 2019 Apr 1;216(4):728-742. doi: 10.1084/jem.20180871. Epub 2019 Feb 27. J Exp Med. 2019. PMID: 30814299 Free PMC article.
-
The mesenchymal context in inflammation, immunity and cancer.Nat Immunol. 2020 Sep;21(9):974-982. doi: 10.1038/s41590-020-0741-2. Epub 2020 Aug 3. Nat Immunol. 2020. PMID: 32747813 Review.
References
-
- Balázs M, Martin F, Zhou T, Kearney JF. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. - PubMed
-
- Kang YS, et al. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. - PubMed
-
- Yuan JS, Kousis PC, Suliman S, Visan I, Guidos CJ. Functions of notch signaling in the immune system: consensus and controversies. Annu. Rev. Immunol. 2010;28:343–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

