Staging the axilla in breast cancer patients with ¹⁸F-FDG PET: how small are the metastases that we can detect with new generation clinical PET systems?
- PMID: 24562642
- PMCID: PMC4006125
- DOI: 10.1007/s00259-014-2689-7
Staging the axilla in breast cancer patients with ¹⁸F-FDG PET: how small are the metastases that we can detect with new generation clinical PET systems?
Abstract
Purpose: Point spread function (PSF) reconstruction improves spatial resolution throughout the entire field of view of a PET system and can detect smaller metastatic deposits than conventional algorithms such as OSEM. We assessed the impact of PSF reconstruction on quantitative values and diagnostic accuracy for axillary staging of breast cancer patients, compared with an OSEM reconstruction, with emphasis on the size of nodal metastases.
Methods: This was a prospective study in a single referral centre in which 50 patients underwent an (18)F-FDG PET examination before axillary lymph node dissection. PET data were reconstructed with an OSEM algorithm and PSF reconstruction, analysed blindly and validated by a pathologist who measured the largest nodal metastasis per axilla. This size was used to evaluate PET diagnostic performance.
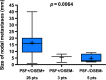
Results: On pathology, 34 patients (68%) had nodal involvement. Overall, the median size of the largest nodal metastasis per axilla was 7 mm (range 0.5 - 40 mm). PSF reconstruction detected more involved nodes than OSEM reconstruction (p = 0.003). The mean PSF to OSEM SUVmax ratio was 1.66 (95 % CI 1.01 - 2.32). The sensitivities of PSF and OSEM reconstructions were, respectively, 96% and 92% in patients with a largest nodal metastasis of >7 mm, 60% and 40% in patients with a largest nodal metastasis of ≤7 mm, and 92% and 69% in patients with a primary tumour ≤30 mm. Biggerstaff graphical comparison showed that globally PSF reconstruction was superior to OSEM reconstruction. The median sizes of the largest nodal metastasis in patients with nodal involvement not detected by either PSF or OSEM reconstruction, detected by PSF but not by OSEM reconstruction and detected by both reconstructions were 3, 6 and 16 mm (p = 0.0064) respectively. In patients with nodal involvement detected by PSF reconstruction but not by OSEM reconstruction, the smallest detectable metastasis was 1.8 mm.
Conclusion: As a result of better activity recovery, PET with PSF reconstruction performed better than PET with OSEM reconstruction in detecting nodal metastases ≤7 mm. However, its sensitivity is still insufficient for it to replace surgical approaches for axillary staging. PET with PSF reconstruction could be used to perform sentinel node biopsy more safely in patients with a primary tumour ≤30 mm and with unremarkable PET results in the axilla.
Figures





Similar articles
-
Clinical evaluation of (18)F-fludeoxyglucose positron emission tomography/CT using point spread function reconstruction for nodal staging of colorectal cancer.Br J Radiol. 2016 Jul;89(1063):20150938. doi: 10.1259/bjr.20150938. Epub 2016 May 5. Br J Radiol. 2016. PMID: 27146065 Free PMC article.
-
Dual time point 2-deoxy-2-[18F]fluoro-D-glucose PET/CT: nodal staging in locally advanced breast cancer.Rev Esp Med Nucl Imagen Mol. 2014 Jan-Feb;33(1):1-5. doi: 10.1016/j.remn.2013.03.005. Epub 2013 May 23. Rev Esp Med Nucl Imagen Mol. 2014. PMID: 23707190 Clinical Trial.
-
Impact of point spread function reconstruction on thoracic lymph node staging with 18F-FDG PET/CT in non-small cell lung cancer.Clin Nucl Med. 2012 Oct;37(10):971-6. doi: 10.1097/RLU.0b013e318251e3d1. Clin Nucl Med. 2012. PMID: 22899197
-
FDG-PET for axillary lymph node staging in primary breast cancer.Eur J Nucl Med Mol Imaging. 2004 Jun;31 Suppl 1:S97-102. doi: 10.1007/s00259-004-1531-z. Epub 2004 May 5. Eur J Nucl Med Mol Imaging. 2004. PMID: 15133635 Review.
-
FDG-PET/CT in the staging of local/regional metastases in breast cancer.Breast. 2011 Dec;20(6):491-4. doi: 10.1016/j.breast.2011.07.002. Epub 2011 Jul 31. Breast. 2011. PMID: 21807517 Review.
Cited by
-
18F-FDG PET/CT heterogeneity quantification through textural features in the era of harmonisation programs: a focus on lung cancer.Eur J Nucl Med Mol Imaging. 2016 Dec;43(13):2324-2335. doi: 10.1007/s00259-016-3441-2. Epub 2016 Jun 21. Eur J Nucl Med Mol Imaging. 2016. PMID: 27325312
-
Implications of reconstruction protocol for histo-biological characterisation of breast cancers using FDG-PET radiomics.EJNMMI Res. 2018 Dec 29;8(1):114. doi: 10.1186/s13550-018-0466-5. EJNMMI Res. 2018. PMID: 30594961 Free PMC article.
-
A nomogram for predicting three or more axillary lymph node involvement before breast cancer surgery.Sci Rep. 2022 Jul 15;12(1):12141. doi: 10.1038/s41598-022-16538-z. Sci Rep. 2022. PMID: 35840785 Free PMC article.
-
Diagnostic performance of 18F-FDG PET/CT using point spread function reconstruction on initial staging of rectal cancer: a comparison study with conventional PET/CT and pelvic MRI.Cancer Imaging. 2018 Jan 30;18(1):4. doi: 10.1186/s40644-018-0137-9. Cancer Imaging. 2018. PMID: 29378659 Free PMC article.
-
In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging.Nat Commun. 2015 Jun 30;6:7560. doi: 10.1038/ncomms8560. Nat Commun. 2015. PMID: 26123615 Free PMC article.
References
-
- Koolen BB, Pengel KE, Wesseling J, Vogel WV, Vrancken Peeters MJ, Vincent AD, et al. Sequential (18)F-FDG PET/CT for early prediction of complete pathological response in breast and axilla during neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:32–40. doi: 10.1007/s00259-013-2515-7. - DOI - PubMed
-
- Straver ME, Aukema TS, Olmos RA, Rutgers EJ, Gilhuijs KG, Schot ME, et al. Feasibility of FDG PET/CT to monitor the response of axillary lymph node metastases to neoadjuvant chemotherapy in breast cancer patients. Eur J Nucl Med Mol Imaging. 2010;37:1069–1076. doi: 10.1007/s00259-009-1343-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

