The Seattle Post Myocardial Infarction Model (SPIM): prediction of mortality after acute myocardial infarction with left ventricular dysfunction
- PMID: 24562803
- PMCID: PMC3932772
- DOI: 10.1177/2048872613502283
The Seattle Post Myocardial Infarction Model (SPIM): prediction of mortality after acute myocardial infarction with left ventricular dysfunction
Abstract
Aims: Ischemic heart disease is a leading worldwide cause of death. The Seattle Post Myocardial Infarction Model (SPIM) was developed to predict survival 6 months to 2 years after an acute myocardial infarction with evidence of left ventricular dysfunction.
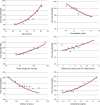
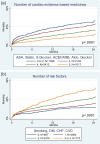
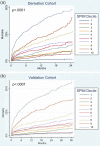
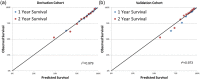
Methods and results: A total of 6632 subjects from the EPHESUS trial were used to derive the predictive model, while 5477 subjects from the OPTIMAAL trial were used to validate the model. Cox proportional hazards modeling was used to develop a multivariate risk score predictive of all-cause mortality. The SPIM risk score integrated lab and vital parameters, Killip class, reperfusion or revascularization, the number of cardiac evidence-based medicines (aspirin, statin, β blocker, ACEI/ARB, aldosterone blocker), and the number of cardiac risk factors. The model was predictive of all-cause mortality after myocardial infarction, with an AUC of 0.75 at 6 months and 0.75 at 2 years in the derivation cohort and 0.77 and 0.78 for the same time points in the validation cohort. Model predicted versus Kaplan-Meier observed survival was excellent in the derivation cohort. It remained so in the validation cohort--84.9% versus 85.0% at 2 years. The 10% of subjects with the highest predicted risk had approximately 25 times higher mortality at 2 years than the 10% of subjects with the lowest predicted risk.
Conclusion: The SPIM score was a powerful predictor of outcomes after myocardial infarction with left ventricular dysfunction. Its highly accurate predictions should improve patient and physician understanding of survival and may prove a useful tool in post-infarct risk stratification.
Keywords: Myocardial infarction; risk model; survival.
Conflict of interest statement
Wayne Levy has received funding from GlaxoSmithKline, Boehringer Ingelheim, and Amgen. Bertram Pitt has received funding from Pfizer, Merck, Novartis, Takeda, Bayer, AstraZeneca, Lilly, BMS, GE Healthcare, Relypsa, BG Medicine, Amorcyte, Cytopherx, Aura Sense, Ardelyx, Forrest Laboratories, and Medtronic.
Figures
References
-
- Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010; 362: 2155–2165 - PubMed
-
- Puymirat E, Simon T, Steg PG, et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA 2012; 308: 998–1006 - PubMed
-
- Jernberg T, Johanson P, Held C, et al. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011; 305: 1677–1684 - PubMed
-
- Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med 2007; 356: 2388–2398 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

