Oligodendroglia in cortical multiple sclerosis lesions decrease with disease progression, but regenerate after repeated experimental demyelination
- PMID: 24563023
- PMCID: PMC4102825
- DOI: 10.1007/s00401-014-1260-8
Oligodendroglia in cortical multiple sclerosis lesions decrease with disease progression, but regenerate after repeated experimental demyelination
Abstract
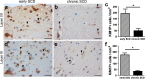
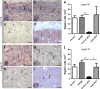
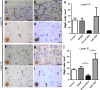
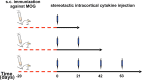
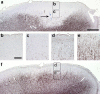
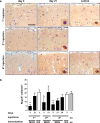
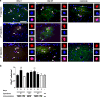
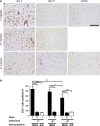
Cerebral cortex shows a high endogenous propensity for remyelination. Yet, widespread subpial cortical demyelination (SCD) is a common feature in progressive multiple sclerosis (MS) and can already be found in early MS. In the present study, we compared oligodendroglial loss in SCD in early and chronic MS. Furthermore, we addressed in an experimental model whether repeated episodes of inflammatory SCD could alter oligodendroglial repopulation and subsequently lead to persistently demyelinated cortical lesions. NogoA(+) mature oligodendrocytes and Olig2(+) oligodendrocyte precursor cells were examined in SCD in patients with early and chronic MS, normal-appearing MS cortex, and control cortex as well as in the rat model of repeated targeted cortical experimental autoimmune encephalomyelitis (EAE). NogoA(+) and Olig2(+) cells were significantly reduced in SCD in patients with chronic, but not early MS. Repeated induction of SCD in rats resulted only in a transient loss of NogoA(+), but not Olig2(+) cells during the demyelination phase. This phase was followed by complete oligodendroglial repopulation and remyelination, even after four episodes of demyelination. Our data indicate efficient oligodendroglial repopulation in subpial cortical lesions in rats after repeated SCD that was similar to early, but not chronic MS cases. Accordingly, four cycles of experimental de- and remyelination were not sufficient to induce sustained remyelination failure as found in chronic cortical MS lesions. This suggests that alternative mechanisms contribute to oligodendrocyte depletion in chronic cortical demyelination in MS.
Figures

Similar articles
-
Early loss of oligodendrocytes in human and experimental neuromyelitis optica lesions.Acta Neuropathol. 2014 Apr;127(4):523-38. doi: 10.1007/s00401-013-1220-8. Epub 2013 Nov 30. Acta Neuropathol. 2014. PMID: 24292009 Free PMC article.
-
Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis.Brain. 2008 Jul;131(Pt 7):1749-58. doi: 10.1093/brain/awn096. Epub 2008 May 30. Brain. 2008. PMID: 18515322
-
Cerebral cortex demyelination and oligodendrocyte precursor response to experimental autoimmune encephalomyelitis.Neurobiol Dis. 2011 Sep;43(3):678-89. doi: 10.1016/j.nbd.2011.05.021. Epub 2011 Jun 6. Neurobiol Dis. 2011. PMID: 21679768
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
-
The response of adult oligodendrocyte progenitors to demyelination in EAE.Prog Brain Res. 2001;132:165-74. doi: 10.1016/s0079-6123(01)32073-3. Prog Brain Res. 2001. PMID: 11544985 Review.
Cited by
-
Glial Response to Intranasal Mesenchymal Stem Cells in Intermittent Cuprizone Model of Demyelination.Neurotox Res. 2022 Oct;40(5):1415-1426. doi: 10.1007/s12640-022-00556-w. Epub 2022 Sep 2. Neurotox Res. 2022. PMID: 36053462
-
An Animal Model for Chronic Meningeal Inflammation and Inflammatory Demyelination of the Cerebral Cortex.Int J Mol Sci. 2023 Sep 9;24(18):13893. doi: 10.3390/ijms241813893. Int J Mol Sci. 2023. PMID: 37762198 Free PMC article.
-
Mechanisms of Demyelination and Remyelination Strategies for Multiple Sclerosis.Int J Mol Sci. 2023 Mar 28;24(7):6373. doi: 10.3390/ijms24076373. Int J Mol Sci. 2023. PMID: 37047344 Free PMC article. Review.
-
Differential contribution of immune effector mechanisms to cortical demyelination in multiple sclerosis.Acta Neuropathol. 2017 Jul;134(1):15-34. doi: 10.1007/s00401-017-1706-x. Epub 2017 Apr 6. Acta Neuropathol. 2017. PMID: 28386765 Free PMC article.
-
Analysis of platelet-derived growth factor receptor A and oligodendrocyte transcription factor 2 markers following Hydroxychloroquine administration in animal induced multiple sclerosis model.Metab Brain Dis. 2021 Oct;36(7):2101-2110. doi: 10.1007/s11011-021-00802-8. Epub 2021 Aug 3. Metab Brain Dis. 2021. PMID: 34342813
References
-
- Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

