Angiopoietin-like protein 2 regulates endothelial colony forming cell vasculogenesis
- PMID: 24563071
- PMCID: PMC4063876
- DOI: 10.1007/s10456-014-9423-8
Angiopoietin-like protein 2 regulates endothelial colony forming cell vasculogenesis
Abstract
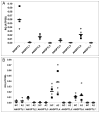
Angiopoietin-like 2 (ANGPTL2) has been reported to induce sprouting angiogenesis; however, its role in vasculogenesis, the de novo lumenization of endothelial cells (EC), remains unexplored. We sought to investigate the potential role of ANGPTL2 in regulating human cord blood derived endothelial colony forming cell (ECFC) vasculogenesis through siRNA mediated inhibition of ANGPTL2 gene expression. We found that ECFCs in which ANGPTL2 was diminished displayed a threefold decrease in in vitro lumenal area whereas addition of exogenous ANGPTL2 protein domains to ECFCs lead to increased lumen formation within a 3 dimensional (3D) collagen assay of vasculogenesis. ECFC migration was attenuated by 36 % via ANGPTL2 knockdown (KD) although proliferation and apoptosis were not affected. We subsequently found that c-Jun NH2-terminal kinase (JNK), but not ERK1/2, phosphorylation was decreased upon ANGPTL2 KD, and expression of membrane type 1 matrix metalloproteinase (MT1-MMP), known to be regulated by JNK and a critical regulator of EC migration and 3D lumen formation, was decreased in lumenized structures in vitro derived from ANGPTL2 silenced ECFCs. Treatment of ECFCs in 3D collagen matrices with either a JNK inhibitor or exogenous rhTIMP-3 (an inhibitor of MT1-MMP activity) resulted in a similar phenotype of decreased vascular lumen formation as observed with ANGPTL2 KD, whereas stimulation of JNK activity increased vasculogenesis. Based on gene silencing, pharmacologic, cellular, and biochemical approaches, we conclude that ANGPTL2 positively regulates ECFC vascular lumen formation likely through its effects on migration and in part by activating JNK and increasing MT1-MMP expression.
Figures



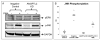


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

