Synthetic lethality in the tobacco plastid ribosome and its rescue at elevated growth temperatures
- PMID: 24563204
- PMCID: PMC3967039
- DOI: 10.1105/tpc.114.123240
Synthetic lethality in the tobacco plastid ribosome and its rescue at elevated growth temperatures
Abstract
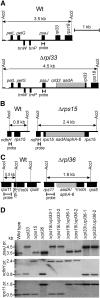
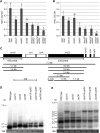
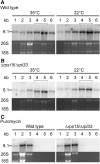
Consistent with their origin from cyanobacteria, plastids (chloroplasts) perform protein biosynthesis on bacterial-type 70S ribosomes. The plastid genomes of seed plants contain a conserved set of ribosomal protein genes. Three of these have proven to be nonessential for translation and, thus, for cellular viability: rps15, rpl33, and rpl36. To help define the minimum ribosome, here, we examined whether more than one of these nonessential plastid ribosomal proteins can be removed from the 70S ribosome. To that end, we constructed all possible double knockouts for the S15, L33, and L36 ribosomal proteins by stable transformation of the tobacco (Nicotiana tabacum) plastid genome. We find that, although S15 and L33 function in different ribosomal particles (30S and 50S, respectively), their combined deletion from the plastid genome results in synthetic lethality under autotrophic conditions. Interestingly, the lethality can be overcome by growth under elevated temperatures due to an improved efficiency of plastid ribosome biogenesis. Our results reveal functional interactions between protein and RNA components of the 70S ribosome and uncover the interdependence of the biogenesis of the two ribosomal subunits. In addition, our findings suggest that defining a minimal set of plastid genes may prove more complex than generally believed.
Figures




Similar articles
-
Rpl33, a nonessential plastid-encoded ribosomal protein in tobacco, is required under cold stress conditions.Plant Cell. 2008 Aug;20(8):2221-37. doi: 10.1105/tpc.108.060392. Epub 2008 Aug 29. Plant Cell. 2008. PMID: 18757552 Free PMC article.
-
Nonessential plastid-encoded ribosomal proteins in tobacco: a developmental role for plastid translation and implications for reductive genome evolution.Plant Cell. 2011 Sep;23(9):3137-55. doi: 10.1105/tpc.111.088906. Epub 2011 Sep 20. Plant Cell. 2011. PMID: 21934145 Free PMC article.
-
The plastid-specific ribosomal proteins of Arabidopsis thaliana can be divided into non-essential proteins and genuine ribosomal proteins.Plant J. 2012 Jan;69(2):302-16. doi: 10.1111/j.1365-313X.2011.04791.x. Epub 2011 Oct 21. Plant J. 2012. PMID: 21923745
-
The translational apparatus of plastids and its role in plant development.Mol Plant. 2014 Jul;7(7):1105-20. doi: 10.1093/mp/ssu022. Epub 2014 Mar 3. Mol Plant. 2014. PMID: 24589494 Free PMC article. Review.
-
Decoding Plant Ribosomal Proteins: Multitasking Players in Cellular Games.Cells. 2025 Mar 21;14(7):473. doi: 10.3390/cells14070473. Cells. 2025. PMID: 40214427 Free PMC article. Review.
Cited by
-
Generation and characterization of a collection of knock-down lines for the chloroplast Clp protease complex in tobacco.J Exp Bot. 2017 Apr 1;68(9):2199-2218. doi: 10.1093/jxb/erx066. J Exp Bot. 2017. PMID: 28369470 Free PMC article.
-
Shine-Dalgarno Sequences Play an Essential Role in the Translation of Plastid mRNAs in Tobacco.Plant Cell. 2017 Dec;29(12):3085-3101. doi: 10.1105/tpc.17.00524. Epub 2017 Nov 13. Plant Cell. 2017. PMID: 29133466 Free PMC article.
-
High-efficiency generation of fertile transplastomic Arabidopsis plants.Nat Plants. 2019 Mar;5(3):282-289. doi: 10.1038/s41477-019-0359-2. Epub 2019 Feb 18. Nat Plants. 2019. PMID: 30778165 Free PMC article.
-
Advancing organelle genome transformation and editing for crop improvement.Plant Commun. 2021 Jan 4;2(2):100141. doi: 10.1016/j.xplc.2021.100141. eCollection 2021 Mar 8. Plant Commun. 2021. PMID: 33898977 Free PMC article. Review.
-
Transgene insertion into the plastid genome alters expression of adjacent native chloroplast genes at the transcriptional and translational levels.Plant Biotechnol J. 2023 Apr;21(4):711-725. doi: 10.1111/pbi.13985. Epub 2023 Jan 17. Plant Biotechnol J. 2023. PMID: 36529916 Free PMC article.
References
-
- Akanuma G., Nanamiya H., Natori Y., Yano K., Suzuki S., Omata S., Ishizuka M., Sekine Y., Kawamura F. (2012). Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation. J. Bacteriol. 194: 6282–6291. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

