Nuclear mechanics in cancer
- PMID: 24563360
- PMCID: PMC4591936
- DOI: 10.1007/978-1-4899-8032-8_20
Nuclear mechanics in cancer
Abstract
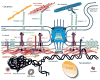
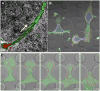
Despite decades of research, cancer metastasis remains an incompletely understood process that is as complex as it is devastating. In recent years, there has been an increasing push to investigate the biomechanical aspects of tumorigenesis, complementing the research on genetic and biochemical changes. In contrast to the high genetic variability encountered in cancer cells, almost all metastatic cells are subject to the same physical constraints as they leave the primary tumor, invade surrounding tissues, transit through the circulatory system, and finally infiltrate new tissues. Advances in live cell imaging and other biophysical techniques, including measurements of subcellular mechanics, have yielded stunning new insights into the physics of cancer cells. While much of this research has been focused on the mechanics of the cytoskeleton and the cellular microenvironment, it is now emerging that the mechanical properties of the cell nucleus and its connection to the cytoskeleton may play a major role in cancer metastasis, as deformation of the large and stiff nucleus presents a substantial obstacle during the passage through the dense interstitial space and narrow capillaries. Here, we present an overview of the molecular components that govern the mechanical properties of the nucleus, and we discuss how changes in nuclear structure and composition observed in many cancers can modulate nuclear mechanics and promote metastatic processes. Improved insights into this interplay between nuclear mechanics and metastatic progression may have powerful implications in cancer diagnostics and therapy and may reveal novel therapeutic targets for pharmacological inhibition of cancer cell invasion.
Figures

References
-
- Fawcett DW. An Atlas of fine structure (The Cell) 2. W. B. Saunders Company; 1966.
-
- Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Käs J, Ulvick S, Bilby C. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88(5):3689–3698. doi: 10.1529/biophysj.104.045476. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

