Menin is required for optimal processing of the microRNA let-7a
- PMID: 24563463
- PMCID: PMC3975034
- DOI: 10.1074/jbc.M113.520692
Menin is required for optimal processing of the microRNA let-7a
Abstract
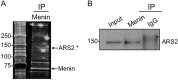
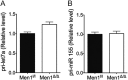
Multiple endocrine neoplasia type I (MEN1) is an inherited syndrome that includes susceptibility to pancreatic islet hyperplasia. This syndrome results from mutations in the MEN1 gene, which encodes menin protein. Menin interacts with several transcription factors, including JunD, and inhibits their activities. However, the precise mechanism by which menin suppresses gene expression is not well understood. Here, we show that menin interacts with arsenite-resistant protein 2 (ARS2), a component of the nuclear RNA CAP-binding complex that is crucial for biogenesis of certain miRNAs including let-7a. The levels of primary-let-7a (pri-let-7a) are not affected by menin; however, the levels of mature let-7a are substantially decreased upon Men1 excision. Let-7a targets, including Insr and Irs2, pro-proliferative genes that are crucial for insulin-mediated signaling, are up-regulated in Men1-excised cells. Inhibition of let-7a using anti-miRNA in wild type cells is sufficient to enhance the expression of insulin receptor substrate 2 (IRS2) to levels observed in Men1-excised cells. Depletion of menin does not affect the expression of Drosha and CBP80, but substantially impairs the processing of pri-miRNA to pre-miRNA. Ars2 knockdown decreased let-7a processing in menin-expressing cells but had little impact on let-7a levels in menin-excised cells. As IRS2 is known to mediate insulin signaling and insulin/mitogen-induced cell proliferation, these findings collectively unravel a novel mechanism whereby menin suppresses cell proliferation, at least partly by promoting the processing of certain miRNAs, including let-7a, leading to suppression of Irs2 expression and insulin signaling.
Keywords: Beta Cell; Cancer Biology; IRS2; Menin; MicroRNA; Pancreas; Pancreatic Islets; Tumor Suppressor Gene; let-7a.
Figures






Similar articles
-
Menin-mediated regulation of miRNA biogenesis uncovers the IRS2 pathway as a target for regulating pancreatic beta cells.Oncoscience. 2014 Sep 15;1(9):562-6. doi: 10.18632/oncoscience.79. eCollection 2014. Oncoscience. 2014. PMID: 25594065 Free PMC article.
-
Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome.Cancer Res. 2013 Apr 15;73(8):2650-8. doi: 10.1158/0008-5472.CAN-12-3158. Epub 2013 Apr 11. Cancer Res. 2013. PMID: 23580576 Free PMC article.
-
Role of miR-24 in Multiple Endocrine Neoplasia Type 1: A Potential Target for Molecular Therapy.Int J Mol Sci. 2021 Jul 8;22(14):7352. doi: 10.3390/ijms22147352. Int J Mol Sci. 2021. PMID: 34298972 Free PMC article. Review.
-
Menin promotes the Wnt signaling pathway in pancreatic endocrine cells.Mol Cancer Res. 2008 Dec;6(12):1894-907. doi: 10.1158/1541-7786.MCR-07-2206. Mol Cancer Res. 2008. PMID: 19074834
-
Menin molecular interactions: insights into normal functions and tumorigenesis.Horm Metab Res. 2005 Jun;37(6):369-74. doi: 10.1055/s-2005-870139. Horm Metab Res. 2005. PMID: 16001329 Review.
Cited by
-
Polymorphisms of microRNA target genes IL12B, INSR, CCND1 and IL10 in gastric cancer.World J Gastroenterol. 2017 May 21;23(19):3480-3487. doi: 10.3748/wjg.v23.i19.3480. World J Gastroenterol. 2017. PMID: 28596683 Free PMC article.
-
β-Cell MicroRNAs: Small but Powerful.Diabetes. 2015 Nov;64(11):3631-44. doi: 10.2337/db15-0831. Diabetes. 2015. PMID: 26494215 Free PMC article. Review.
-
Epigenetic regulation by the menin pathway.Endocr Relat Cancer. 2017 Oct;24(10):T147-T159. doi: 10.1530/ERC-17-0298. Epub 2017 Aug 15. Endocr Relat Cancer. 2017. PMID: 28811300 Free PMC article. Review.
-
Lessons from basic pancreatic beta cell research in type-2 diabetes and vascular complications.Diabetol Int. 2017 Jan 23;8(2):139-152. doi: 10.1007/s13340-017-0304-4. eCollection 2017 Jun. Diabetol Int. 2017. PMID: 30603317 Free PMC article.
-
MicroRNA-142-3p, a novel target of tumor suppressor menin, inhibits osteosarcoma cell proliferation by down-regulation of FASN.Tumour Biol. 2014 Oct;35(10):10287-93. doi: 10.1007/s13277-014-2316-z. Epub 2014 Jul 18. Tumour Biol. 2014. Retraction in: Tumour Biol. 2017 Apr 20. doi: 10.1007/s13277-017-5487-6. PMID: 25034529 Retracted.
References
-
- Libé R., Bertherat J. (2005) Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur. J. Endocrinol. 153, 477–487 - PubMed
-
- Burgess J. R., Nord B., David R., Greenaway T. M., Parameswaran V., Larsson C., Shepherd J. J., Teh B. T. (2000) Phenotype and phenocopy: the relationship between genotype and clinical phenotype in a single large family with multiple endocrine neoplasia type 1 (MEN 1). Clin. Endocrinol. 53, 205–211 - PubMed
-
- Marx S. J. (2005) Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat. Rev. Cancer 5, 367–375 - PubMed
-
- Bertolino P., Radovanovic I., Casse H., Aguzzi A., Wang Z. Q., Zhang C. X. (2003) Genetic ablation of the tumor suppressor menin causes lethality at mid-gestation with defects in multiple organs. Mech. Dev. 120, 549–560 - PubMed
-
- Chandrasekharappa S. C., Guru S. C., Manickam P., Olufemi S. E., Collins F. S., Emmert-Buck M. R., Debelenko L. V., Zhuang Z., Lubensky I. A., Liotta L. A., Crabtree J. S., Wang Y., Roe B. A., Weisemann J., Boguski M. S., Agarwal S. K., Kester M. B., Kim Y. S., Heppner C., Dong Q., Spiegel A. M., Burns A. L., Marx S. J. (1997) Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276, 404–407 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

