Direct regulation of osteocytic connexin 43 hemichannels through AKT kinase activated by mechanical stimulation
- PMID: 24563481
- PMCID: PMC4036178
- DOI: 10.1074/jbc.M114.550608
Direct regulation of osteocytic connexin 43 hemichannels through AKT kinase activated by mechanical stimulation
Abstract
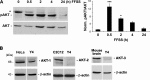
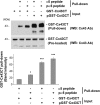
Connexin (Cx) 43 hemichannels in osteocytes are thought to play a critical role in releasing bone modulators in response to mechanical loading, a process important for bone formation and remodeling. However, the underlying mechanism that regulates the opening of mechanosensitive hemichannels is largely unknown. We have recently shown that Cx43 and integrin α5 interact directly with each other, and activation of PI3K appears to be required for Cx43 hemichannel opening by mechanical stimulation. Here, we show that mechanical loading through fluid flow shear stress (FFSS) increased the level of active AKT, a downstream effector of PI3K, which is correlated with the opening of hemichannels. Both Cx43 and integrin α5 are directly phosphorylated by AKT. Inhibition of AKT activation significantly reduced FFSS-induced opening of hemichannels and disrupted the interaction between Cx43 and integrin α5. Moreover, AKT phosphorylation on Cx43 and integrin α5 enhanced their interaction. In contrast to the C terminus of wild-type Cx43, overexpression of the C-terminal mutant containing S373A, a consensus site previously shown to be phosphorylated by AKT, failed to bind with α5 and hence could not inhibit hemichannel opening. Together, our results suggest that AKT activated by FFSS directly phosphorylates Cx43 and integrin α5, and Ser-373 of Cx43 plays a predominant role in mediating the interaction between these two proteins and Cx43 hemichannel opening, a crucial step to mediate the anabolic function of mechanical loading in the bone.
Keywords: Akt; Connexin; Integrin; Mechanotransduction; Phosphorylation.
Figures






References
-
- Aarden E. M., Burger E. H., Nijweide P. J. (1994) Function of osteocytes in bone. J. Cell. Biochem. 55, 287–299 - PubMed
-
- Burger E. H., Klein-Nulend J. (1999) Mechanotransduction in bone: role of the lacunocanalicular network. FASEB J. 13, S101–112 - PubMed
-
- Cowin S. C., Moss-Salentijin L., Moss M. L. (1991) Candidates for the mechanosensory system in bone. J. Biomed. Eng. 113, 191–197 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

