Fibroblast growth factor-based signaling through synthetic heparan sulfate blocks copolymers studied using high cell density three-dimensional cell printing
- PMID: 24563485
- PMCID: PMC3975022
- DOI: 10.1074/jbc.M113.546937
Fibroblast growth factor-based signaling through synthetic heparan sulfate blocks copolymers studied using high cell density three-dimensional cell printing
Abstract
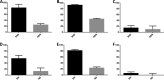
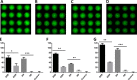
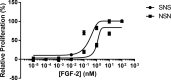
Four well-defined heparan sulfate (HS) block copolymers containing S-domains (high sulfo group content) placed adjacent to N-domains (low sulfo group content) were chemoenzymatically synthesized and characterized. The domain lengths in these HS block co-polymers were ~40 saccharide units. Microtiter 96-well and three-dimensional cell-based microarray assays utilizing murine immortalized bone marrow (BaF3) cells were developed to evaluate the activity of these HS block co-polymers. Each recombinant BaF3 cell line expresses only a single type of fibroblast growth factor receptor (FGFR) but produces neither HS nor fibroblast growth factors (FGFs). In the presence of different FGFs, BaF3 cell proliferation showed clear differences for the four HS block co-polymers examined. These data were used to examine the two proposed signaling models, the symmetric FGF2-HS2-FGFR2 ternary complex model and the asymmetric FGF2-HS1-FGFR2 ternary complex model. In the symmetric FGF2-HS2-FGFR2 model, two acidic HS chains bind in a basic canyon located on the top face of the FGF2-FGFR2 protein complex. In this model the S-domains at the non-reducing ends of the two HS proteoglycan chains are proposed to interact with the FGF2-FGFR2 protein complex. In contrast, in the asymmetric FGF2-HS1-FGFR2 model, a single HS chain interacts with the FGF2-FGFR2 protein complex through a single S-domain that can be located at any position within an HS chain. Our data comparing a series of synthetically prepared HS block copolymers support a preference for the symmetric FGF2-HS2-FGFR2 ternary complex model.
Keywords: Block Copolymers; Fibroblast Growth Factor (FGF); Fibroblast Growth Factor Receptor (FGFR); Fibroblast Growth Factors; Glycosaminoglycan; Heparan Sulfate; Polysaccharide; Signaling Complex; Three-dimensional Cellular Printing.
Figures

Similar articles
-
Heparan Sulfate Domains Required for Fibroblast Growth Factor 1 and 2 Signaling through Fibroblast Growth Factor Receptor 1c.J Biol Chem. 2017 Feb 10;292(6):2495-2509. doi: 10.1074/jbc.M116.761585. Epub 2016 Dec 28. J Biol Chem. 2017. PMID: 28031461 Free PMC article.
-
Highly sulfated nonreducing end-derived heparan sulfate domains bind fibroblast growth factor-2 with high affinity and are enriched in biologically active fractions.J Biol Chem. 2011 Jun 3;286(22):19311-9. doi: 10.1074/jbc.M110.204693. Epub 2011 Apr 6. J Biol Chem. 2011. PMID: 21471211 Free PMC article.
-
Fibroblast growth factor receptors 1 and 2 interact differently with heparin/heparan sulfate. Implications for dynamic assembly of a ternary signaling complex.J Biol Chem. 2002 Aug 9;277(32):28554-63. doi: 10.1074/jbc.M111754200. Epub 2002 May 28. J Biol Chem. 2002. PMID: 12034712
-
A protein canyon in the FGF-FGF receptor dimer selects from an à la carte menu of heparan sulfate motifs.Curr Opin Struct Biol. 2005 Oct;15(5):506-16. doi: 10.1016/j.sbi.2005.09.002. Curr Opin Struct Biol. 2005. PMID: 16154740 Review.
-
Insights into the role of heparan sulphate in fibroblast growth factor signalling.Biochem Soc Trans. 2006 Jun;34(Pt 3):442-5. doi: 10.1042/BST0340442. Biochem Soc Trans. 2006. PMID: 16709182 Review.
Cited by
-
Can we produce heparin/heparan sulfate biomimetics using "mother-nature" as the gold standard?Molecules. 2015 Mar 5;20(3):4254-76. doi: 10.3390/molecules20034254. Molecules. 2015. PMID: 25751786 Free PMC article. Review.
-
Glycosaminoglycan-Based Biohybrid Hydrogels: A Sweet and Smart Choice for Multifunctional Biomaterials.Adv Mater. 2016 Oct;28(40):8861-8891. doi: 10.1002/adma.201601908. Epub 2016 Jul 27. Adv Mater. 2016. PMID: 27461855 Free PMC article. Review.
-
Potential Use of Anti-Inflammatory Synthetic Heparan Sulfate to Attenuate Liver Damage.Biomedicines. 2020 Nov 16;8(11):503. doi: 10.3390/biomedicines8110503. Biomedicines. 2020. PMID: 33207634 Free PMC article. Review.
-
Fell-Muir Lecture: Heparan sulphate and the art of cell regulation: a polymer chain conducts the protein orchestra.Int J Exp Pathol. 2015 Aug;96(4):203-31. doi: 10.1111/iep.12135. Epub 2015 Jul 15. Int J Exp Pathol. 2015. PMID: 26173450 Free PMC article.
-
Glycosaminoglycans: What Remains To Be Deciphered?JACS Au. 2023 Mar 2;3(3):628-656. doi: 10.1021/jacsau.2c00569. eCollection 2023 Mar 27. JACS Au. 2023. PMID: 37006755 Free PMC article. Review.
References
-
- Caterson B., Flannery C. R., Hughes C. E., Little C. B. (2000) Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 19, 333–344 - PubMed
-
- Kresse H., Schönherr E. (2001) Proteoglycans of the extracellular matrix and growth control. J. Cell. Physiol. 189, 266–274 - PubMed
-
- Sasisekharan R., Venkataraman G. (2000) Heparin and heparan sulfate. Biosynthesis, structure, and function. Curr. Opin. Chem. Biol. 4, 626–631 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R42 ES018022/ES/NIEHS NIH HHS/United States
- R01 HL096972/HL/NHLBI NIH HHS/United States
- R41 ES018022/ES/NIEHS NIH HHS/United States
- R01 GM038060/GM/NIGMS NIH HHS/United States
- R01 HL094463/HL/NHLBI NIH HHS/United States
- HL62244/HL/NHLBI NIH HHS/United States
- HL096972/HL/NHLBI NIH HHS/United States
- U01 GM102137/GM/NIGMS NIH HHS/United States
- ES 020903/ES/NIEHS NIH HHS/United States
- GM38060/GM/NIGMS NIH HHS/United States
- R01 ES020903/ES/NIEHS NIH HHS/United States
- ES 018022/ES/NIEHS NIH HHS/United States
- R01 HL062244/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

