A novel aminotetralin-type serotonin (5-HT) 2C receptor-specific agonist and 5-HT2A competitive antagonist/5-HT2B inverse agonist with preclinical efficacy for psychoses
- PMID: 24563531
- PMCID: PMC3989798
- DOI: 10.1124/jpet.113.212373
A novel aminotetralin-type serotonin (5-HT) 2C receptor-specific agonist and 5-HT2A competitive antagonist/5-HT2B inverse agonist with preclinical efficacy for psychoses
Erratum in
- J Pharmacol Exp Ther. 2014 Jun 1;349(3):533
Abstract
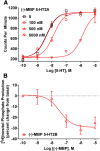
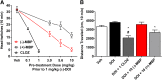
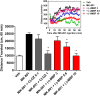
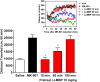
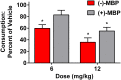
Development of 5-HT2C agonists for treatment of neuropsychiatric disorders, including psychoses, substance abuse, and obesity, has been fraught with difficulties, because the vast majority of reported 5-HT2C selective agonists also activate 5-HT2A and/or 5-HT2B receptors, potentially causing hallucinations and/or cardiac valvulopathy. Herein is described a novel, potent, and efficacious human 5-HT2C receptor agonist, (-)-trans-(2S,4R)-4-(3'[meta]-bromophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine (-)-MBP), that is a competitive antagonist and inverse agonist at human 5-HT2A and 5-HT2B receptors, respectively. (-)-MBP has efficacy comparable to the prototypical second-generation antipsychotic drug clozapine in three C57Bl/6 mouse models of drug-induced psychoses: the head-twitch response elicited by [2,5]-dimethoxy-4-iodoamphetamine; hyperlocomotion induced by MK-801 [(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (dizocilpine maleate)]; and hyperlocomotion induced by amphetamine. (-)-MBP, however, does not alter locomotion when administered alone, distinguishing it from clozapine, which suppresses locomotion. Finally, consumption of highly palatable food by mice was not increased by (-)-MBP at a dose that produced at least 50% maximal efficacy in the psychoses models. Compared with (-)-MBP, the enantiomer (+)-MBP was much less active across in vitro affinity and functional assays using mouse and human receptors and also translated in vivo with comparably lower potency and efficacy. Results indicate a 5-HT2C receptor-specific agonist, such as (-)-MBP, may be pharmacotherapeutic for psychoses, without liability for obesity, hallucinations, heart disease, sedation, or motoric disorders.
Figures


Similar articles
-
Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model.Neuropharmacology. 2013 Jul;70:112-21. doi: 10.1016/j.neuropharm.2013.01.007. Epub 2013 Jan 23. Neuropharmacology. 2013. PMID: 23353901 Free PMC article.
-
Development of homogeneous high-affinity agonist binding assays for 5-HT2 receptor subtypes.Assay Drug Dev Technol. 2005 Dec;3(6):649-59. doi: 10.1089/adt.2005.3.649. Assay Drug Dev Technol. 2005. PMID: 16438660
-
(1R, 3S)-(-)-trans-PAT: a novel full-efficacy serotonin 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist/antagonist activity.Eur J Pharmacol. 2009 Aug 1;615(1-3):1-9. doi: 10.1016/j.ejphar.2009.04.035. Epub 2009 May 3. Eur J Pharmacol. 2009. PMID: 19397907 Free PMC article.
-
Predictive in silico studies of human 5-hydroxytryptamine receptor subtype 2B (5-HT2B) and valvular heart disease.Curr Top Med Chem. 2013;13(11):1353-62. doi: 10.2174/15680266113139990039. Curr Top Med Chem. 2013. PMID: 23675941 Free PMC article. Review.
-
Targeting the 5-HT2C Receptor in Biological Context and the Current State of 5-HT2C Receptor Ligand Development.Curr Top Med Chem. 2019;19(16):1381-1398. doi: 10.2174/1568026619666190709101449. Curr Top Med Chem. 2019. PMID: 31288724 Free PMC article. Review.
Cited by
-
Pimavanserin and Lorcaserin Attenuate Measures of Binge Eating in Male Sprague-Dawley Rats.Front Pharmacol. 2018 Dec 7;9:1424. doi: 10.3389/fphar.2018.01424. eCollection 2018. Front Pharmacol. 2018. PMID: 30581386 Free PMC article.
-
Studies on the antifungal and serotonin receptor agonist activities of the secondary metabolites from piezotolerant deep-sea fungus Ascotricha sp.Mycology. 2018 Nov 21;10(2):92-108. doi: 10.1080/21501203.2018.1541934. eCollection 2019 Jun. Mycology. 2018. PMID: 31069123 Free PMC article.
-
The synthetic cathinone psychostimulant α-PPP antagonizes serotonin 5-HT2A receptors: In vitro and in vivo evidence.Drug Test Anal. 2019 Jul;11(7):990-998. doi: 10.1002/dta.2582. Epub 2019 Apr 22. Drug Test Anal. 2019. PMID: 30845376 Free PMC article.
-
Calcium and Neural Stem Cell Proliferation.Int J Mol Sci. 2024 Apr 6;25(7):4073. doi: 10.3390/ijms25074073. Int J Mol Sci. 2024. PMID: 38612887 Free PMC article. Review.
-
Serotonin-2C receptor agonists decrease potassium-stimulated GABA release in the nucleus accumbens.Synapse. 2015 Feb;69(2):78-85. doi: 10.1002/syn.21790. Epub 2014 Nov 20. Synapse. 2015. PMID: 25382408 Free PMC article.
References
-
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. (1998) Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 155:761–767 - PubMed
-
- Aghajanian GK, Marek GJ. (2000) Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev 31:302–312 - PubMed
-
- Alex KD, Yavanian GJ, McFarlane HG, Pluto CP, Pehek EA. (2005) Modulation of dopamine release by striatal 5-HT2C receptors. Synapse 55:242–251 - PubMed
-
- Arena Pharmaceuticals (2012) Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQ® (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182 ed).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

