Synthesis, characterisation and evaluation of N-mannich bases of 2-substituted Benzimidazole derivatives
- PMID: 24563595
- PMCID: PMC3930114
- DOI: 10.1016/j.jyp.2013.11.004
Synthesis, characterisation and evaluation of N-mannich bases of 2-substituted Benzimidazole derivatives
Abstract
Rationale: Benzimidazoles and its derivatives represent one of the mainly biological active classes of literature.
Aim: In this present study aimed to synthesize N-mannich bases derivatives compounds bearing of 2-substituted benzimidazole moiety, in order to investigate their possible biological activity.
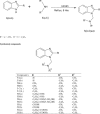
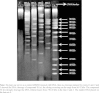
Method: Benzimidazole compounds were prepared from the condensation reaction between ortho phenylene diamine and various acids. Mannich base of newly synthesized Benzimidazole derivatives were synthesized from 2-substituted Benzimidazoles by reacting with secondary amines. The purity of the compounds was ascertained by melting point (m.p) and thin layer chromatography (TLC). Structures of the synthesized compounds were elucidated by spectral data. Antimicrobial assay was performed by microbroth dilution method. Bacterial genomic DNA cleavage was assessed by Agarose gel electrophoresis. Toxicity of the most effective compounds was studied by Brine-shrimp lethality assay.
Result: Among the synthesized compounds, compound 5E (a) and (b) was establish to be the most potent against all tested microorganisms. This two compounds exhibited complete bacterial DNA cleavage and non-toxic.
Conclusion: These results suggest that it an interesting compound compared to the current therapeutic agents and are considered to investigate further for the same.
Keywords: Benzimidazoles; Brine-shrimp lethality; Mannich base; Microbroth dilution; O-Phenylene diamine.
Figures
References
-
- Kalyankar T.M., Pekamwar S.S., Wadher S.J., Tiprale P.S., Shinde G.H. Review on benzimidazole derivative. Int J Che Pharm Sci. 2012;3:1–10.
-
- Elerafi Mohamed G., Ibrahim Mohamed N. Synthesis and spectral studies of mannich bases derived from 2-substituted benzimidazoles. Int J Chem Tech Res. 2010;2:2097–2099.
-
- Langtry H.D., Wilde M.I. Omeprazole: a review of its use in Helicobacter pylori infection, gastro-oesophageal reflux disease and peptic ulcers induced by non-steroidal anti-inflammatory drugs. Drugs. 1998;56:447–486. - PubMed
-
- Hazelton J.C., Iddon B., Suschitzky H., Woolley L.H. Synthesis of polysubstituted o-phenylenediamines and their conversion into heterocycles, particularly 2-substituted benzimidazoles with known or potential anthelminthic activity. Tetrahedron. 1995;51:10771–10794.
-
- Ansari K.F., Lal C. Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur J Med Chem. 2009;44:4028–4033. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources