Parallel mechanisms for direct and indirect membrane protein trafficking by synucleins
- PMID: 24563712
- PMCID: PMC3917945
- DOI: 10.4161/cib.26794
Parallel mechanisms for direct and indirect membrane protein trafficking by synucleins
Abstract
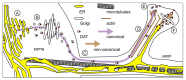
More than 2 decades of work have yet to conclusively determine the physiological role of the synuclein proteins, even though these abundant brain constituents are participants in a broad array of cellular processes. Among proposed physiological roles is a functional interaction between the synuclein proteins and monoamine transporters contributing to transporter trafficking through direct protein-protein interactions. Recent work shows that an antagonistic effect of the synuclein proteins on the secretory functions of the endoplasmic reticulum and the Golgi apparatus appears to simultaneously influence trafficking of the dopamine transporter and other membrane proteins. Here, we highlight these new findings in view of the broader literature identifying the role of synucleins in protein trafficking and suggest emerging themes for ongoing and future work in the field of synuclein biology.
Keywords: Golgi; Parkinson’s disease; dopamine uptake; endoplasmic reticulum; mood disorders; synuclein; trafficking.
Figures
Similar articles
-
Synucleins antagonize endoplasmic reticulum function to modulate dopamine transporter trafficking.PLoS One. 2013 Aug 13;8(8):e70872. doi: 10.1371/journal.pone.0070872. eCollection 2013. PLoS One. 2013. PMID: 23967127 Free PMC article.
-
Synuclein modulation of monoamine transporters.FEBS Lett. 2011 Apr 6;585(7):1001-6. doi: 10.1016/j.febslet.2011.03.009. Epub 2011 Mar 23. FEBS Lett. 2011. PMID: 21396366 Free PMC article. Review.
-
Trypsin disrupts the trafficking of the human dopamine transporter by alpha-synuclein and its A30P mutant.Biochemistry. 2004 Feb 10;43(5):1242-53. doi: 10.1021/bi035308s. Biochemistry. 2004. PMID: 14756560
-
Alpha-synuclein Toxicity in the Early Secretory Pathway: How It Drives Neurodegeneration in Parkinsons Disease.Front Neurosci. 2015 Nov 12;9:433. doi: 10.3389/fnins.2015.00433. eCollection 2015. Front Neurosci. 2015. PMID: 26617485 Free PMC article. Review.
-
Mutations in the lipid-binding domain of alpha-synuclein confer overlapping, yet distinct, functional properties in the regulation of dopamine transporter activity.Mol Cell Neurosci. 2003 Sep;24(1):91-105. doi: 10.1016/s1044-7431(03)00124-6. Mol Cell Neurosci. 2003. PMID: 14550771
Cited by
-
Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice.Int J Mol Sci. 2022 Feb 4;23(3):1807. doi: 10.3390/ijms23031807. Int J Mol Sci. 2022. PMID: 35163729 Free PMC article.
-
Sequence determinants of the Caenhorhabditis elegans dopamine transporter dictating in vivo axonal export and synaptic localization.Mol Cell Neurosci. 2017 Jan;78:41-51. doi: 10.1016/j.mcn.2016.11.011. Epub 2016 Nov 30. Mol Cell Neurosci. 2017. PMID: 27913309 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
