Acetaminophen-induced liver damage in mice is associated with gender-specific adduction of peroxiredoxin-6
- PMID: 24563856
- PMCID: PMC3926121
- DOI: 10.1016/j.redox.2014.01.008
Acetaminophen-induced liver damage in mice is associated with gender-specific adduction of peroxiredoxin-6
Abstract
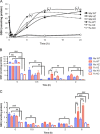
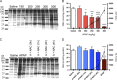
The mechanism by which acetaminophen (APAP) causes liver damage evokes many aspects drug metabolism, oxidative chemistry, and genetic-predisposition. In this study, we leverage the relative resistance of female C57BL/6 mice to APAP-induced liver damage (AILD) compared to male C57BL/6 mice in order to identify the cause(s) of sensitivity. Furthermore, we use mice that are either heterozygous (HZ) or null (KO) for glutamate cysteine ligase modifier subunit (Gclm), in order to titrate the toxicity relative to wild-type (WT) mice. Gclm is important for efficient de novo synthesis of glutathione (GSH). APAP (300 mg/kg, ip) or saline was administered and mice were collected at 0, 0.5, 1, 2, 6, 12, and 24 h. Male mice showed marked elevation in serum alanine aminotransferase by 6 h. In contrast, female WT and HZ mice showed minimal toxicity at all time points. Female KO mice, however, showed AILD comparable to male mice. Genotype-matched male and female mice showed comparable APAP-protein adducts, with Gclm KO mice sustaining significantly greater adducts. ATP was depleted in mice showing toxicity, suggesting impaired mitochondria function. Indeed, peroxiredoxin-6, a GSH-dependent peroxiredoxin, was preferentially adducted by APAP in mitochondria of male mice but rarely adducted in female mice. These results support parallel mechanisms of toxicity where APAP adduction of peroxiredoxin-6 and sustained GSH depletion results in the collapse of mitochondria function and hepatocyte death. We conclude that adduction of peroxiredoxin-6 sensitizes male C57BL/6 mice to toxicity by acetaminophen.
Keywords: Acetaminophen; Gclm; Gender; Glutathione; Mitochondria; Peroxiredoxin-6.
Figures






References
-
- Botta D., Shi S., White C.C., Dabrowski M.J., Keener C.L., Srinouanprachanh S.L., Farin F.M., Ware C.B., Ladiges W.C., Pierce R.H., Fausto N., Kavanagh T.J. Acetaminophen-induced liver injury is attenuated in male glutamate–cysteine ligase transgenic mice. J. Biol. Chem. 2006;281:28865–28875. - PubMed
-
- Braun L., Kardon T., Puskas F., Csala M., Banhegyi G., Mandl J. Regulation of glucuronidation by glutathione redox state through the alteration of UDP-glucose supply originating from glycogen metabolism. Arch. Biochem. Biophys. 1997;348:169–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01ES10849/ES/NIEHS NIH HHS/United States
- P01 AG001751/AG/NIA NIH HHS/United States
- T32 AG000057/AG/NIA NIH HHS/United States
- T32AG000057/AG/NIA NIH HHS/United States
- P42 ES004696/ES/NIEHS NIH HHS/United States
- P30ES007033/ES/NIEHS NIH HHS/United States
- T32 ES007032/ES/NIEHS NIH HHS/United States
- P30 ES007033/ES/NIEHS NIH HHS/United States
- R01 ES010849/ES/NIEHS NIH HHS/United States
- P42ES004696/ES/NIEHS NIH HHS/United States
- T32 GM007750/GM/NIGMS NIH HHS/United States
- T32ES007032/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

