Thiol-based H2O2 signalling in microbial systems
- PMID: 24563858
- PMCID: PMC3926117
- DOI: 10.1016/j.redox.2014.01.015
Thiol-based H2O2 signalling in microbial systems
Abstract
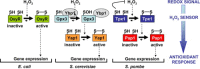
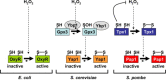
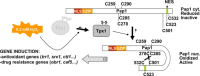
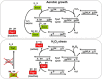
Cysteine residues, and in particular their thiolate groups, react not only with reactive oxygen species but also with electrophiles and with reactive nitrogen species. Thus, cysteine oxidation has often been linked to the toxic effects of some of these reactive molecules. However, thiol-based switches are common in protein sensors of antioxidant cascades, in both prokaryotic and eukaryotic organisms. We will describe here three redox sensors, the transcription factors OxyR, Yap1 and Pap1, which respond by disulfide bond formation to hydrogen peroxide stress, focusing specially on the differences among the three peroxide-sensing mechanisms.
Keywords: Cys oxidation; H2O2 sensor; OxyR; Pap1; S. pombe; Yap1.
Figures
References
-
- Kadokura H., Katzen F., Beckwith J. Protein disulfide bond formation in prokaryotes. Annu. Rev. Biochem. 2003;72:111–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

