Epigenetic modification, dehydration, and molecular crowding effects on the thermodynamics of i-motif structure formation from C-rich DNA
- PMID: 24564458
- PMCID: PMC3985701
- DOI: 10.1021/bi401523b
Epigenetic modification, dehydration, and molecular crowding effects on the thermodynamics of i-motif structure formation from C-rich DNA
Abstract
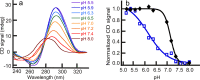
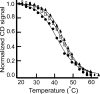
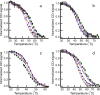
DNA sequences with the potential to form secondary structures such as i-motifs (iMs) and G-quadruplexes (G4s) are abundant in the promoters of several oncogenes and, in some instances, are known to regulate gene expression. Recently, iM-forming DNA strands have also been employed as functional units in nanodevices, ranging from drug delivery systems to nanocircuitry. To understand both the mechanism of gene regulation by iMs and how to use them more efficiently in nanotechnological applications, it is essential to have a thorough knowledge of factors that govern their conformational states and stabilities. Most of the prior work to characterize the conformational dynamics of iMs have been done with iM-forming synthetic constructs like tandem (CCT)n repeats and in standard dilute buffer systems. Here, we present a systematic study on the consequences of epigenetic modifications, molecular crowding, and degree of hydration on the stabilities of an iM-forming sequence from the promoter of the c-myc gene. Our results indicate that 5-hydroxymethylation of cytosines destabilized the iMs against thermal and pH-dependent melting; contrarily, 5-methylcytosine modification stabilized the iMs. Under molecular crowding conditions (PEG-300, 40% w/v), the thermal stability of iMs increased by ∼10 °C, and the pKa was raised from 6.1 ± 0.1 to 7.0 ± 0.1. Lastly, the iM's stability at varying degrees of hydration in 1,2-dimethoxyethane, 2-methoxyethanol, ethylene glycol, 1,3-propanediol, and glycerol cosolvents indicated that the iMs are stabilized by dehydration because of the release of water molecules when folded. Our results highlight the importance of considering the effects of epigenetic modifications, molecular crowding, and the degree of hydration on iM structural dynamics. For example, the incorporation of 5-methylycytosines and 5-hydroxymethlycytosines in iMs could be useful for fine-tuning the pH- or temperature-dependent folding/unfolding of an iM. Variations in the degree of hydration of iMs may also provide an additional control of the folded/unfolded state of iMs without having to change the pH of the surrounding matrix.
Figures


References
-
- Moehlig A. R.; Djernes K. E.; Krishnan V. M.; Hooley R. J. (2012) Cytosine derivatives form hemiprotonated dimers in solution and the gas phase. Org. Lett. 14, 2560–2563. - PubMed
-
- Choi J.; Kim S.; Tachikawa T.; Fujitsuka M.; Majima T. (2011) pH-induced intramolecular folding dynamics of i-motif DNA. J. Am. Chem. Soc. 133, 16146–16153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

