Tbx1 modulates endodermal and mesodermal differentiation from mouse induced pluripotent stem cells
- PMID: 24564535
- PMCID: PMC4066228
- DOI: 10.1089/scd.2013.0488
Tbx1 modulates endodermal and mesodermal differentiation from mouse induced pluripotent stem cells
Abstract
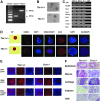
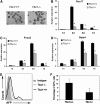
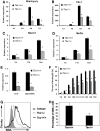
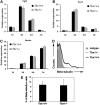
The T-box transcriptional factor (Tbx) family of transcriptional factors has distinct roles in a wide range of embryonic differentiation or response pathways. Tbx1, a T-box transcription factor, is an important gene for the human congenital disorder 22q11.2 deletion syndrome. Induced pluripotent stem cell (iPSC) technology offers new opportunities for both elucidation of the pathogenesis of diseases and the development of stem-cell-based therapies. In this study, we generated iPSCs from Tbx1(-/-) and Tbx1(+/+) fibroblasts and investigated the spontaneous differentiation potential of iPSCs by detailed lineage analysis of the iPSC-derived embryoid bodies. Undifferentiated Tbx1(-/-) and Tbx1(+/+) iPSCs showed similar expression levels of pluripotent markers. The ability of the Tbx1(-/-) iPSCs to generate endodermal and mesodermal lineages was compromised upon spontaneous differentiation into embryonic bodies. Restoration of Tbx1 expression in the Tbx1(-/-) iPSCs to normal levels using an inducible lentiviral system rescued these cells from the potential of defective differentiation. Interestingly, overexpression of Tbx1 in the Tbx1(-/-) iPSCs to higher levels than in the Tbx1(+/+) iPSCs again led to a defective differentiation potential. Additionally, we observed that expression of fibroblast growth factor (FGF) 10 and FGF8 was downregulated in the Tbx1(-/-) iPSC-derived cells, which suggests that Tbx1 regulates the expression of FGFs. Taken together, our results implicated the Tbx1 level as an important determinant of endodermal and mesodermal lineage differentiation during embryonic development.
Figures


Similar articles
-
Early thyroid development requires a Tbx1-Fgf8 pathway.Dev Biol. 2009 Apr 1;328(1):109-17. doi: 10.1016/j.ydbio.2009.01.014. Epub 2009 Jan 20. Dev Biol. 2009. PMID: 19389367 Free PMC article.
-
Variation in mesodermal and hematopoietic potential of adult skin-derived induced pluripotent stem cell lines in mice.Stem Cell Rev Rep. 2011 Nov;7(4):958-68. doi: 10.1007/s12015-011-9249-3. Stem Cell Rev Rep. 2011. PMID: 21424235
-
Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development.Development. 2006 Sep;133(18):3587-95. doi: 10.1242/dev.02539. Epub 2006 Aug 16. Development. 2006. PMID: 16914493 Free PMC article.
-
Induced pluripotent stem cells and hepatic differentiation.J Chin Med Assoc. 2013 Nov;76(11):599-605. doi: 10.1016/j.jcma.2013.07.007. Epub 2013 Aug 9. J Chin Med Assoc. 2013. PMID: 23933345 Review.
-
[Induction and differentiation of induced pluripotent stem cells into macrophages: a review].Sheng Wu Gong Cheng Xue Bao. 2021 Nov 25;37(11):4001-4014. doi: 10.13345/j.cjb.210134. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 34841800 Review. Chinese.
Cited by
-
T-Box20 inhibits osteogenic differentiation in adipose-derived human mesenchymal stem cells: the role of T-Box20 on osteogenesis.J Biol Res (Thessalon). 2019 Sep 18;26:8. doi: 10.1186/s40709-019-0099-5. eCollection 2019 Dec. J Biol Res (Thessalon). 2019. PMID: 31548928 Free PMC article.
-
CHD7 regulates definitive endodermal and mesodermal development from human embryonic stem cells.Stem Cell Res Ther. 2025 Jun 17;16(1):311. doi: 10.1186/s13287-025-04437-9. Stem Cell Res Ther. 2025. PMID: 40528267 Free PMC article.
-
Recombinant FOXN1 fusion protein increases T cell generation in old mice.Front Immunol. 2024 Jul 12;15:1423488. doi: 10.3389/fimmu.2024.1423488. eCollection 2024. Front Immunol. 2024. PMID: 39072332 Free PMC article.
-
c-Met signalling is required for efficient postnatal thymic regeneration and repair.Immunology. 2015 Feb;144(2):245-53. doi: 10.1111/imm.12365. Immunology. 2015. PMID: 25074726 Free PMC article.
-
Skint8, a Novel B7 Family-Related Molecule, Negatively Regulates T Cell Responses.J Immunol. 2019 Jul 15;203(2):400-407. doi: 10.4049/jimmunol.1800639. Epub 2019 Jun 12. J Immunol. 2019. PMID: 31189570 Free PMC article.
References
-
- Takahashi K. and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 - PubMed
-
- Li W, Wang D, Qin J, Liu C, Zhang Q, Zhang X, Yu X, Lahn BT, Mao FF. and Xiang AP. (2010). Generation of functional hepatocytes from mouse induced pluripotent stem cells. J Cell Physiol 222:492–501 - PubMed
-
- Naiche LA, Harrelson Z, Kelly RG. and Papaioannou VE. (2005). T-box genes in vertebrate development. Annu Rev Genet 39:219–239 - PubMed
-
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM. and Papaioannou VE. (1996). Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn 206:379–390 - PubMed
-
- Vitelli F, Morishima M, Taddei I, Lindsay EA. and Baldini A. (2002). Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum Mol Genet 11:915–922 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

