Neurovascular unit on a chip: implications for translational applications
- PMID: 24564885
- PMCID: PMC4029462
- DOI: 10.1186/scrt379
Neurovascular unit on a chip: implications for translational applications
Abstract
The blood-brain barrier (BBB) dynamically controls exchange between the brain and the body, but this interaction cannot be studied directly in the intact human brain or sufficiently represented by animal models. Most existing in vitro BBB models do not include neurons and glia with other BBB elements and do not adequately predict drug efficacy and toxicity. Under the National Institutes of Health Microtissue Initiative, we are developing a three-dimensional, multicompartment, organotypic microphysiological system representative of a neurovascular unit of the brain. The neurovascular unit system will serve as a model to study interactions between the central nervous system neurons and the cerebral spinal fluid (CSF) compartment, all coupled to a realistic blood-surrogate supply and venous return system that also incorporates circulating immune cells and the choroid plexus. Hence all three critical brain barriers will be recapitulated: blood-brain, brain-CSF, and blood-CSF. Primary and stem cell-derived human cells will interact with a variety of agents to produce critical chemical communications across the BBB and between brain regions. Cytomegalovirus, a common herpesvirus, will be used as an initial model of infections regulated by the BBB. This novel technological platform, which combines innovative microfluidics, cell culture, analytical instruments, bioinformatics, control theory, neuroscience, and drug discovery, will replicate chemical communication, molecular trafficking, and inflammation in the brain. The platform will enable targeted and clinically relevant nutritional and pharmacologic interventions for or prevention of such chronic diseases as obesity and acute injury such as stroke, and will uncover potential adverse effects of drugs. If successful, this project will produce clinically useful technologies and reveal new insights into how the brain receives, modifies, and is affected by drugs, other neurotropic agents, and diseases.
Figures
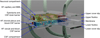
References
-
- Wikswo JP, Block FE III, Cliffel DE, Goodwin CR, Marasco CC, Markov DA, McLean DL, McLean JA, McKenzie JR, Reiserer RS, Samson PC, Schaffer DK, Seale KT, Sherrod SD. Engineering challenges for instrumenting and controlling integrated organ-on-a-chip systems. IEEE Trans Biomed Eng. 2013;4:682–690. - PMC - PubMed
-
- Integrated Microphysiological Systems for Drug Efficacy and Toxicity Testing in Human Health and Disease (UH2/UH3) http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-11-022.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

