Promyelocytic leukemia zinc-finger induction signs mesenchymal stem cell commitment: identification of a key marker for stemness maintenance?
- PMID: 24564963
- PMCID: PMC4055047
- DOI: 10.1186/scrt416
Promyelocytic leukemia zinc-finger induction signs mesenchymal stem cell commitment: identification of a key marker for stemness maintenance?
Abstract
Introduction: Mesenchymal stem cells (MSCs) are an attractive cell source for cartilage and bone tissue engineering given their ability to differentiate into chondrocytes and osteoblasts. However, the common origin of these two specialized cell types raised the question about the identification of regulatory pathways determining the differentiation fate of MSCs into chondrocyte or osteoblast.
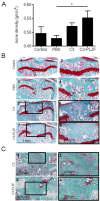
Methods: Chondrogenesis, osteoblastogenesis, and adipogenesis of human and mouse MSC were induced by using specific inductive culture conditions. Expression of promyelocytic leukemia zinc-finger (PLZF) or differentiation markers in MSCs was determined by RT-qPCR. PLZF-expressing MSC were implanted in a mouse osteochondral defect model and the neotissue was analyzed by routine histology and microcomputed tomography.
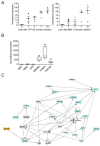
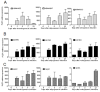
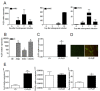
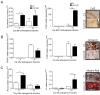
Results: We found out that PLZF is not expressed in MSCs and its expression at early stages of MSC differentiation is the mark of their commitment toward the three main lineages. PLZF acts as an upstream regulator of both Sox9 and Runx2, and its overexpression in MSC enhances chondrogenesis and osteogenesis while it inhibits adipogenesis. In vivo, implantation of PLZF-expressing MSC in mice with full-thickness osteochondral defects resulted in the formation of a reparative tissue resembling cartilage and bone.
Conclusions: Our findings demonstrate that absence of PLZF is required for stemness maintenance and its expression is an early event at the onset of MSC commitment during the differentiation processes of the three main lineages.
Figures

Similar articles
-
Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue.Stem Cell Res Ther. 2017 Dec 6;8(1):275. doi: 10.1186/s13287-017-0716-x. Stem Cell Res Ther. 2017. PMID: 29208029 Free PMC article.
-
Role of RHEB in Regulating Differentiation Fate of Mesenchymal Stem Cells for Cartilage and Bone Regeneration.Int J Mol Sci. 2017 Apr 24;18(4):880. doi: 10.3390/ijms18040880. Int J Mol Sci. 2017. PMID: 28441755 Free PMC article.
-
SOX9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells.Stem Cell Res Ther. 2012;3(3):22. doi: 10.1186/scrt113. Stem Cell Res Ther. 2012. PMID: 22742415 Free PMC article.
-
Current topics in pharmacological research on bone metabolism: Promyelotic leukemia zinc finger (PLZF) and tumor necrosis factor-alpha-stimulated gene 6 (TSG-6) identified by gene expression analysis play roles in the pathogenesis of ossification of the posterior longitudinal ligament.J Pharmacol Sci. 2006 Mar;100(3):205-10. doi: 10.1254/jphs.fmj05004x5. J Pharmacol Sci. 2006. PMID: 16547399 Review.
-
Promyelocytic leukemia zinc finger controls type 2 immune responses in the lungs by regulating lineage commitment and the function of innate and adaptive immune cells.Int Immunopharmacol. 2024 Mar 30;130:111670. doi: 10.1016/j.intimp.2024.111670. Epub 2024 Feb 18. Int Immunopharmacol. 2024. PMID: 38373386 Review.
Cited by
-
PLZF targets developmental enhancers for activation during osteogenic differentiation of human mesenchymal stem cells.Elife. 2019 Jan 23;8:e40364. doi: 10.7554/eLife.40364. Elife. 2019. PMID: 30672466 Free PMC article.
-
BMP and Hedgehog Regulate Distinct AGM Hematopoietic Stem Cells Ex Vivo.Stem Cell Reports. 2016 Mar 8;6(3):383-95. doi: 10.1016/j.stemcr.2016.01.016. Epub 2016 Feb 25. Stem Cell Reports. 2016. PMID: 26923823 Free PMC article.
-
Identification of Novel EZH2 Targets Regulating Osteogenic Differentiation in Mesenchymal Stem Cells.Stem Cells Dev. 2016 Jun 15;25(12):909-21. doi: 10.1089/scd.2015.0384. Epub 2016 Jun 7. Stem Cells Dev. 2016. PMID: 27168161 Free PMC article.
-
Tbx4 function during hindlimb development reveals a mechanism that explains the origins of proximal limb defects.Development. 2021 Oct 1;148(19):dev199580. doi: 10.1242/dev.199580. Epub 2021 Sep 24. Development. 2021. PMID: 34423345 Free PMC article.
References
-
- Ullah M, Stich S, Notter M, Eucker J, Sittinger M, Ringe J. Transdifferentiation of mesenchymal stem cells-derived adipogenic-differentiated cells into osteogenic- or chondrogenic-differentiated cells proceeds via dedifferentiation and have a correlation with cell cycle arresting and driving genes. Differentiation. 2013;85:78–90. doi: 10.1016/j.diff.2013.02.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

