24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS)
- PMID: 24565357
- PMCID: PMC3982864
- DOI: 10.1016/j.jaac.2013.11.010
24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS)
Abstract
Objective: We report active treatment group differences on response and remission rates and changes in anxiety severity at weeks 24 and 36 for the Child/Adolescent Anxiety Multimodal Study (CAMS).
Method: CAMS youth (N = 488; 74% ≤ 12 years of age) with DSM-IV separation, generalized, or social anxiety disorder were randomized to 12 weeks of cognitive-behavioral therapy (CBT), sertraline (SRT), CBT+SRT (COMB), or medication management/pill placebo (PBO). Responders attended 6 monthly booster sessions in their assigned treatment arm; youth in COMB and SRT continued on their medication throughout this period. Efficacy of COMB, SRT, and CBT (n = 412) was assessed at 24 and 36 weeks postrandomization. Youth randomized to PBO (n = 76) were offered active CAMS treatment if nonresponsive at week 12 or over follow-up and were not included here. Independent evaluators blind to study condition assessed anxiety severity, functioning, and treatment response. Concomitant treatments were allowed but monitored over follow-up.
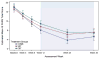
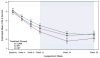
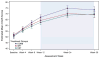
Results: The majority (>80%) of acute responders maintained positive response at both weeks 24 and 36. Consistent with acute outcomes, COMB maintained advantage over CBT and SRT, which did not differ, on dimensional outcomes; the 3 treatments did not differ on most categorical outcomes over follow-up. Compared to COMB and CBT, youth in SRT obtained more concomitant psychosocial treatments, whereas those in SRT and CBT obtained more concomitant combined (medication plus psychosocial) treatment.
Conclusions: COMB maintained advantage over CBT and SRT on some measures over follow-up, whereas the 2 monotherapies remained indistinguishable. The observed convergence of COMB and monotherapy may be related to greater use of concomitant treatment during follow-up among youth receiving the monotherapies, although other explanations are possible. Although outcomes were variable, most CAMS-treated youth experienced sustained treatment benefit. Clinical trial registration information-Child and Adolescent Anxiety Disorders (CAMS); URL: http://clinicaltrials.gov. Unique identifier: NCT00052078.
Keywords: Child/Adolescent Anxiety Multimodal Study (CAMS); anxiety; cognitive-behavioral therapy (CBT); follow-up; selective serotonin reuptake inhibitor (SSRI).
Copyright © 2014 American Academy of Child and Adolescent Psychiatry. All rights reserved.
Figures

Comment in
-
Treating anxiety in youth: does maintenance treatment maintain?J Am Acad Child Adolesc Psychiatry. 2014 Mar;53(3):269-70. doi: 10.1016/j.jaac.2013.11.008. J Am Acad Child Adolesc Psychiatry. 2014. PMID: 24565354 No abstract available.
References
-
- Costello J, Egger H, Angold A. The developmental epidemiology of anxiety disorders: Phenomenology, prevalence, and Comorbidity. Child Adol Clinics North America. 2005;14:631–648. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

