Severe asthma: an expanding and mounting clinical challenge
- PMID: 24565450
- PMCID: PMC4880055
- DOI: 10.1016/j.jaip.2013.01.005
Severe asthma: an expanding and mounting clinical challenge
Abstract
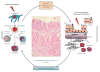
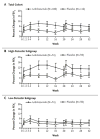
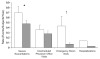
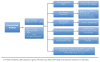
Although all patients with asthma have variable airflow obstruction, airway inflammation, and bronchial hyperresponsiveness, some have disease that is severe in many aspects: persistent airflow obstruction, ongoing symptoms, increased frequency of exacerbations, and, most importantly, a diminished response to medications. A number of definitions have emerged to characterize the clinical features of severe asthma, but a central feature of this phenotype is the need for high doses of medications, especially corticosteroids, in attempts to achieve disease control. The prevalence of severe asthma is also undergoing reevaluation from the usual estimate of 10% to larger numbers on the basis of medication needs and the lack of disease control achieved. At present, the underlying mechanisms of severe asthma are not established but likely reflect a heterogeneous pattern, rather than a single unifying process. Guideline-directed treatment for severe asthma has limits with usual approaches centered on high doses of inhaled corticosteroids, long-acting β-agonists, and trials with omalizumab, the monoclonal antibody to IgE. With the development of approaches to recognize asthma phenotypes with distinct pathogenesis and hence unique therapeutic targets, it is hoped that a personalized strategy in treatment directed toward disease-specific features will improve outcomes for this high-risk, severely affected population of patients.
Keywords: Asthma; anti-IgE; bronchodilators; corticosteroids; immunomodulators.
Copyright © 2013 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012:1–8. - PubMed
-
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–44. - PubMed
-
- World Health Organization. Office of Health Communications and Public Relations. Asthma. Geneva: World Health Organization; 2006.
-
- Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126:926–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

