Telatinib reverses chemotherapeutic multidrug resistance mediated by ABCG2 efflux transporter in vitro and in vivo
- PMID: 24565910
- PMCID: PMC3983711
- DOI: 10.1016/j.bcp.2014.02.012
Telatinib reverses chemotherapeutic multidrug resistance mediated by ABCG2 efflux transporter in vitro and in vivo
Abstract
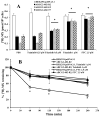
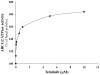
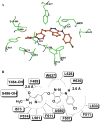
Multidrug resistance (MDR) is a phenomenon where cancer cells become simultaneously resistant to anticancer drugs with different structures and mechanisms of action. MDR has been shown to be associated with overexpression of ATP-binding cassette (ABC) transporters. Here, we report that telatinib, a small molecule tyrosine kinase inhibitor, enhances the anticancer activity of ABCG2 substrate anticancer drugs by inhibiting ABCG2 efflux transporter activity. Co-incubation of ABCG2-overexpressing drug resistant cell lines with telatinib and ABCG2 substrate anticancer drugs significantly reduced cellular viability, whereas telatinib alone did not significantly affect drug sensitive and drug resistant cell lines. Telatinib at 1 μM did not significantly alter the expression of ABCG2 in ABCG2-overexpressing cell lines. Telatinib at 1 μM significantly enhanced the intracellular accumulation of [(3)H]-mitoxantrone (MX) in ABCG2-overexpressing cell lines. In addition, telatinib at 1 μM significantly reduced the rate of [(3)H]-MX efflux from ABCG2-overexpressing cells. Furthermore, telatinib significantly inhibited ABCG2-mediated transport of [(3)H]-E₂17βG in ABCG2 overexpressing membrane vesicles. Telatinib stimulated the ATPase activity of ABCG2 in a concentration-dependent manner, indicating that telatinib might be a substrate of ABCG2. Binding interactions of telatinib were found to be in transmembrane region of homology modeled human ABCG2. In addition, telatinib (15 mg/kg) with doxorubicin (1.8 mg/kg) significantly decreased the growth rate and tumor size of ABCG2 overexpressing tumors in a xenograft nude mouse model. These results, provided that they can be translated to humans, suggesting that telatinib, in combination with specific ABCG2 substrate drugs may be useful in treating tumors that overexpress ABCG2.
Keywords: ABC transporter; ABCG2; Multidrug resistance; Telatinib; Tyrosine kinase inhibitor.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2.Cancer Res. 2008 Oct 1;68(19):7905-14. doi: 10.1158/0008-5472.CAN-08-0499. Cancer Res. 2008. PMID: 18829547 Free PMC article.
-
Dacomitinib potentiates the efficacy of conventional chemotherapeutic agents via inhibiting the drug efflux function of ABCG2 in vitro and in vivo.J Exp Clin Cancer Res. 2018 Feb 20;37(1):31. doi: 10.1186/s13046-018-0690-x. J Exp Clin Cancer Res. 2018. PMID: 29458405 Free PMC article.
-
Reversal effect of FW-04-806, a macrolide dilactone compound, on multidrug resistance mediated by ABCB1 and ABCG2 in vitro and in vivo.Cell Commun Signal. 2019 Sep 1;17(1):110. doi: 10.1186/s12964-019-0408-5. Cell Commun Signal. 2019. PMID: 31472682 Free PMC article.
-
Drug transporters: recent advances concerning BCRP and tyrosine kinase inhibitors.Br J Cancer. 2008 Mar 11;98(5):857-62. doi: 10.1038/sj.bjc.6604213. Epub 2008 Feb 5. Br J Cancer. 2008. PMID: 18253130 Free PMC article. Review.
-
Multidrug resistance in cancer chemotherapy and xenobiotic protection mediated by the half ATP-binding cassette transporter ABCG2.Curr Med Chem Anticancer Agents. 2004 Jan;4(1):31-42. doi: 10.2174/1568011043482205. Curr Med Chem Anticancer Agents. 2004. PMID: 14754410 Review.
Cited by
-
Cabozantinib Reverses Topotecan Resistance in Human Non-Small Cell Lung Cancer NCI-H460/TPT10 Cell Line and Tumor Xenograft Model.Front Cell Dev Biol. 2021 Mar 22;9:640957. doi: 10.3389/fcell.2021.640957. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33829017 Free PMC article.
-
MBL-II-141, a chromone derivative, enhances irinotecan (CPT-11) anticancer efficiency in ABCG2-positive xenografts.Oncotarget. 2014 Dec 15;5(23):11957-70. doi: 10.18632/oncotarget.2566. Oncotarget. 2014. PMID: 25474134 Free PMC article.
-
Tyrosine kinase inhibitors as reversal agents for ABC transporter mediated drug resistance.Molecules. 2014 Sep 4;19(9):13848-77. doi: 10.3390/molecules190913848. Molecules. 2014. PMID: 25191874 Free PMC article. Review.
-
Optimization Of Cancer Treatment Through Overcoming Drug Resistance.J Cancer Res Oncobiol. 2018;1(2):107. doi: 10.31021/jcro.20181107. Epub 2018 Feb 27. J Cancer Res Oncobiol. 2018. PMID: 29932172 Free PMC article.
-
Esters of the marine-derived triterpene sipholenol A reverse P-GP-mediated drug resistance.Mar Drugs. 2015 Apr 14;13(4):2267-86. doi: 10.3390/md13042267. Mar Drugs. 2015. PMID: 25874923 Free PMC article.
References
-
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. - PubMed
-
- Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–99. - PubMed
-
- Gottesman MM, Fojo T, Bates SE. Multidrug Resistance in Cancer: Role of Atp-Dependent Transporters. Nature Reviews Cancer. 2002;2:48–58. - PubMed
-
- Dean M. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Research. 2001;11:1156–66. - PubMed
-
- Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

