Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421
- PMID: 24565955
- PMCID: PMC3982883
- DOI: 10.1093/jnci/dju013
Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421
Abstract
Background: Prior studies suggest that elevated markers of bone turnover are prognostic for poor survival in castration-resistant prostate cancer (CRPC). The predictive role of these markers relative to bone-targeted therapy is unknown. We prospectively evaluated the prognostic and predictive value of bone biomarkers in sera from CRPC patients treated on a placebo-controlled phase III trial of docetaxel with or without the bone targeted endothelin-A receptor antagonist atrasentan (SWOG S0421).
Methods: Markers for bone resorption (N-telopeptide and pyridinoline) and formation (C-terminal collagen propeptide and bone alkaline phosphatase) were assayed in pretreatment and serial sera. Cox proportional hazards regression models were fit for overall survival. Models were fit with main effects for marker levels and with/without terms for marker-treatment interaction, adjusted for clinical variables, to assess the prognostic and predictive value of atrasentan. Analysis was adjusted for multiple comparisons. Two-sided P values were calculated using the Wald test.
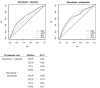
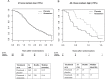
Results: Sera from 778 patients were analyzed. Elevated baseline levels of each of the markers were associated with worse survival (P < .001). Increasing marker levels by week nine of therapy were also associated with subsequent poor survival (P < .001). Patients with the highest marker levels (upper 25th percentile for all markers) not only had a poor prognosis (hazard ratio [HR] = 4.3; 95% confidence interval [CI] = 2.41 to 7.65; P < .001) but also had a survival benefit from atrasentan (HR = 0.33; 95% CI = 0.15 to 0.71; median survival = 13 [atrasentan] vs 5 months [placebo]; P interaction = .005).
Conclusions: Serum bone metabolism markers have statistically significant independent prognostic value in CRPC. Importantly, a small group of patients (6%) with highly elevated markers of bone turnover appear to preferentially benefit from atrasentan therapy.
Trial registration: ClinicalTrials.gov NCT00134056.
Figures

Similar articles
-
Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer.J Clin Oncol. 2014 Apr 10;32(11):1136-42. doi: 10.1200/JCO.2013.51.7417. Epub 2014 Mar 10. J Clin Oncol. 2014. PMID: 24616308 Free PMC article. Clinical Trial.
-
Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial.Lancet Oncol. 2013 Aug;14(9):893-900. doi: 10.1016/S1470-2045(13)70294-8. Epub 2013 Jul 17. Lancet Oncol. 2013. PMID: 23871417 Free PMC article. Clinical Trial.
-
Serum alkaline phosphatase differentiates prostate-specific antigen flare from early disease progression after docetaxel chemotherapy in castration-resistant prostate cancer with bone metastasis.J Cancer Res Clin Oncol. 2014 Oct;140(10):1769-76. doi: 10.1007/s00432-014-1710-7. Epub 2014 May 24. J Cancer Res Clin Oncol. 2014. PMID: 24858569 Free PMC article.
-
Present, Emerging and Possible Future Biomarkers in Castration Resistant Prostate Cancer (CRPC).Curr Cancer Drug Targets. 2015;15(3):243-55. doi: 10.2174/1568009615666150204145803. Curr Cancer Drug Targets. 2015. PMID: 25654638 Review.
-
ZD4054: a specific endothelin A receptor antagonist with promising activity in metastatic castration-resistant prostate cancer.Expert Opin Investig Drugs. 2008 Aug;17(8):1237-45. doi: 10.1517/13543784.17.8.1237. Expert Opin Investig Drugs. 2008. PMID: 18616419 Free PMC article. Review.
Cited by
-
Biomarkers for the Management of Castration-Resistant Prostate Cancer: We Are Not There Yet.Target Oncol. 2017 Aug;12(4):401-412. doi: 10.1007/s11523-017-0500-y. Target Oncol. 2017. PMID: 28620691 Review.
-
Markers of bone metabolism and overall survival in men with bone-metastatic hormone sensitive prostate cancer (HSPC): A subset analysis of SWOG S1216, a phase III trial of androgen deprivation with or without orteronel.Prostate Cancer Prostatic Dis. 2024 Sep;27(3):566-570. doi: 10.1038/s41391-024-00813-3. Epub 2024 Feb 29. Prostate Cancer Prostatic Dis. 2024. PMID: 38424319 Free PMC article. Clinical Trial.
-
Castration Determines the Efficacy of ETAR Blockade in a Mouse Model of Prostate Cancer Bone Metastasis.Endocrinology. 2019 Aug 1;160(8):1786-1796. doi: 10.1210/en.2019-00261. Endocrinology. 2019. PMID: 31173072 Free PMC article.
-
Biomarkers for Treatment Response in Advanced Prostate Cancer.Cancers (Basel). 2021 Nov 16;13(22):5723. doi: 10.3390/cancers13225723. Cancers (Basel). 2021. PMID: 34830878 Free PMC article. Review.
-
The best of both worlds - managing the cancer, saving the bone.Nat Rev Endocrinol. 2016 Jan;12(1):29-42. doi: 10.1038/nrendo.2015.185. Epub 2015 Oct 27. Nat Rev Endocrinol. 2016. PMID: 26503674 Free PMC article. Review.
References
-
- Pollen JJ, Gerber K, Ashburn WL, et al. Nuclear bone imaging in metastatic cancer of the prostate. Cancer. 1981;47 (11):2585–2594 - PubMed
-
- Maeda H, Koizumi M, Yoshimura K, et al. Correlation between bone metabolic markers and bone scan in prostatic cancer. J Urol. 1997;157 (2):539–543 - PubMed
-
- Fontana A, Delmas PD. Markers of bone turnover in bone metastases. Cancer. 2000;88 (12 suppl):295–2960 - PubMed
-
- Ali SM, Demers LM, Leitzel K, et al. Baseline serum NTx levels are prognostic in metastatic breast cancer patients with bone-only metastasis. Ann Oncol. 2004;15 (3):455–459 - PubMed
-
- Lara PN, Jr, Stadler WM, Longmate J, et al. A randomized phase II trial of the matrix metalloproteinase inhibitor BMS-275291 in hormone-refractory prostate cancer patients with bone metastases. Clin Cancer Res. 2006;12(5):1556–1563 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

