Functional transformations of bile acid transporters induced by high-affinity macromolecules
- PMID: 24566561
- PMCID: PMC3933907
- DOI: 10.1038/srep04163
Functional transformations of bile acid transporters induced by high-affinity macromolecules
Erratum in
-
Corrigendum: Functional transformations of bile acid transporters induced by high-affinity macromolecules.Sci Rep. 2015 Aug 5;5:9407. doi: 10.1038/srep09407. Sci Rep. 2015. PMID: 26244565 Free PMC article. No abstract available.
Abstract
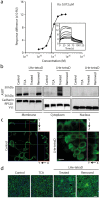
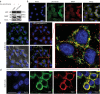
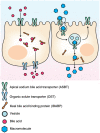
Apical sodium-dependent bile acid transporters (ASBT) are the intestinal transporters that form intermediate complexes with substrates and its conformational change drives the movement of substrates across the cell membrane. However, membrane-based intestinal transporters are confined to the transport of only small molecular substrates. Here, we propose a new strategy that uses high-affinity binding macromolecular substrates to functionally transform the membrane transporters so that they behave like receptors, ultimately allowing the apical-basal transport of bound macromolecules. Bile acid based macromolecular substrates were synthesized and allowed to interact with ASBT. ASBT/macromolecular substrate complexes were rapidly internalized in vesicles, localized in early endosomes, dissociated and escaped the vesicular transport while binding of cytoplasmic ileal bile acid binding proteins cause exocytosis of macromolecules and prevented entry into lysosomes. This newly found transformation process of ASBT suggests a new transport mechanism that could aid in further utilization of ASBT to mediate oral macromolecular drug delivery.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

