Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo
- PMID: 24566629
- PMCID: PMC3993459
- DOI: 10.1128/IAI.00087-14
Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo
Abstract
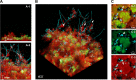
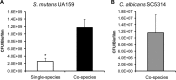
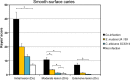
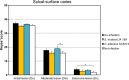
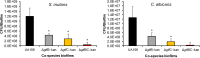
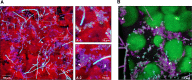
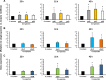
Streptococcus mutans is often cited as the main bacterial pathogen in dental caries, particularly in early-childhood caries (ECC). S. mutans may not act alone; Candida albicans cells are frequently detected along with heavy infection by S. mutans in plaque biofilms from ECC-affected children. It remains to be elucidated whether this association is involved in the enhancement of biofilm virulence. We showed that the ability of these organisms together to form biofilms is enhanced in vitro and in vivo. The presence of C. albicans augments the production of exopolysaccharides (EPS), such that cospecies biofilms accrue more biomass and harbor more viable S. mutans cells than single-species biofilms. The resulting 3-dimensional biofilm architecture displays sizeable S. mutans microcolonies surrounded by fungal cells, which are enmeshed in a dense EPS-rich matrix. Using a rodent model, we explored the implications of this cross-kingdom interaction for the pathogenesis of dental caries. Coinfected animals displayed higher levels of infection and microbial carriage within plaque biofilms than animals infected with either species alone. Furthermore, coinfection synergistically enhanced biofilm virulence, leading to aggressive onset of the disease with rampant carious lesions. Our in vitro data also revealed that glucosyltransferase-derived EPS is a key mediator of cospecies biofilm development and that coexistence with C. albicans induces the expression of virulence genes in S. mutans (e.g., gtfB, fabM). We also found that Candida-derived β1,3-glucans contribute to the EPS matrix structure, while fungal mannan and β-glucan provide sites for GtfB binding and activity. Altogether, we demonstrate a novel mutualistic bacterium-fungus relationship that occurs at a clinically relevant site to amplify the severity of a ubiquitous infectious disease.
Figures


References
-
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltran-Aguilar ED, Horowitz AM, Li CH. 2007. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat. 11:1–92 - PubMed
-
- Hallett KB, O'Rourke PK. 2002. Early childhood caries and infant feeding practice. Community Dent. Health 19:237–242 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

