Safety of medical interventions in children versus adults
- PMID: 24567023
- PMCID: PMC9923602
- DOI: 10.1542/peds.2013-3128
Safety of medical interventions in children versus adults
Abstract
Objective: Compare the risk of harm from pharmacologic interventions in pediatric versus adult randomized controlled trials (RCTs).
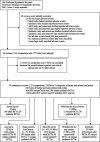
Methods: We used systematic reviews from the Cochrane Database of Systematic Reviews. We considered separately 7 categories of harms/harm-related end points: severe harms, withdrawals due to harms, any harm, organ system-level harms, specific harms, withdrawals for any reason, and mortality. Systematic reviews with quantitative synthesis from at least 1 adult and 1 pediatric RCT for any of those end points were eligible. We calculated the summary odds ratio (experimental versus control intervention) in adult and pediatric trials/meta-analysis; the relative odds ratio (ROR) in adults versus children per meta-analysis; and the summary ROR (sROR) across all meta-analyses for each end point. ROR <1 means that the experimental intervention fared worse in children than adults.
Results: We identified 176 meta-analyses for 52 types of harms/harm-related end points with 669 adult and 184 pediatric RCTs. Of those, 165 had sufficient data for ROR estimation. sRORs showed statistically significant discrepancy between adults and children only for headache (sROR 0.82; 95% confidence interval 0.70-0.96). Nominally significant discrepancies for specific harms were identified in 12 of 165 meta-analyses (RORs <1 in 7, ROR >1 in 5). In 36% of meta-analyses, the ROR estimates suggested twofold or greater differences between children and adults, and the 95% confidence intervals could exclude twofold differences only in 18% of meta-analyses.
Conclusions: Available evidence on harms/harm-related end points from pharmacologic interventions has large uncertainty. Extrapolation of evidence from adults to children may be tenuous. Some clinically important discrepancies were identified.
Keywords: adults; children; comparative safety; harms; mortality; pharmacologic interventions; withdrawals.
Conflict of interest statement
Figures
Similar articles
-
Comparative evidence on harms in pediatric randomized clinical trials from less developed versus more developed countries is limited.J Clin Epidemiol. 2018 Mar;95:63-72. doi: 10.1016/j.jclinepi.2017.11.016. Epub 2017 Nov 28. J Clin Epidemiol. 2018. PMID: 29191447 Review.
-
Comparative rates of harms in randomized trials from more developed versus less developed countries may be different.J Clin Epidemiol. 2016 Oct;78:10-21. doi: 10.1016/j.jclinepi.2016.02.032. Epub 2016 Apr 6. J Clin Epidemiol. 2016. PMID: 27063207
-
Comparative effectiveness of medical interventions in adults versus children.J Pediatr. 2010 Aug;157(2):322-330.e17. doi: 10.1016/j.jpeds.2010.02.011. J Pediatr. 2010. PMID: 20434730
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Physicians' vs patients' global assessments of disease activity in rheumatology and musculoskeletal trials: A meta-research project with focus on reasons for discrepancies.Semin Arthritis Rheum. 2022 Oct;56:152074. doi: 10.1016/j.semarthrit.2022.152074. Epub 2022 Jul 21. Semin Arthritis Rheum. 2022. PMID: 35921746 Review.
Cited by
-
In silico & in vitro approaches suggest osteoclastogenesis induction underlying fractures in Entrectinib-treated children.Arch Toxicol. 2025 Jun 25. doi: 10.1007/s00204-025-04111-2. Online ahead of print. Arch Toxicol. 2025. PMID: 40563023
-
LEVERAGING UNSTRUCTURED DATA IN ELECTRONIC HEALTH RECORDS TO DETECT ADVERSE EVENTS FROM PEDIATRIC DRUG USE - A SCOPING REVIEW.medRxiv [Preprint]. 2025 Mar 20:2025.03.20.25324320. doi: 10.1101/2025.03.20.25324320. medRxiv. 2025. Update in: Annu Rev Biomed Data Sci. 2025 Aug;8(1):227-250. doi: 10.1146/annurev-biodatasci-111224-124530. PMID: 40166566 Free PMC article. Updated. Preprint.
-
Do systematic reviews on pediatric topics need special methodological considerations?BMC Pediatr. 2017 Mar 6;17(1):57. doi: 10.1186/s12887-017-0812-1. BMC Pediatr. 2017. PMID: 28260530 Free PMC article.
-
Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: long term observational follow-up in the Costa Rica HPV Vaccine Trial.BMJ. 2015 Sep 7;351:h4358. doi: 10.1136/bmj.h4358. BMJ. 2015. PMID: 26346155 Free PMC article.
-
Comparison of nuisance parameters in pediatric versus adult randomized trials: a meta-epidemiologic empirical evaluation.BMC Med Res Methodol. 2018 Jan 10;18(1):7. doi: 10.1186/s12874-017-0456-8. BMC Med Res Methodol. 2018. PMID: 29321002 Free PMC article.
References
-
- Cohen AL , Budnitz DS , Weidenbach KN , et al. . National surveillance of emergency department visits for outpatient adverse drug events in children and adolescents. J Pediatr. 2008;152(3):416–421 - PubMed
-
- Holdsworth MT , Fichtl RE , Behta M , et al. . Incidence and impact of adverse drug events in pediatric inpatients. Arch Pediatr Adolesc Med. 2003;157(1):60–65 - PubMed
-
- Taché SV , Sönnichsen A , Ashcroft DM . Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7–8):977–989 - PubMed
-
- Tullus K . Safety concerns of angiotensin II receptor blockers in preschool children. Arch Dis Child. 2011;96(9):881–882 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources