Molecular basis for preventing α-synuclein aggregation by a molecular tweezer
- PMID: 24567327
- PMCID: PMC4036189
- DOI: 10.1074/jbc.M113.524520
Molecular basis for preventing α-synuclein aggregation by a molecular tweezer
Abstract
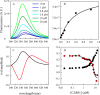
Recent work on α-synuclein has shown that aggregation is controlled kinetically by the rate of reconfiguration of the unstructured chain, such that the faster the reconfiguration, the slower the aggregation. In this work we investigate this relationship by examining α-synuclein in the presence of a small molecular tweezer, CLR01, which binds selectively to Lys side chains. We find strong binding to multiple Lys within the chain as measured by fluorescence and mass-spectrometry and a linear increase in the reconfiguration rate with concentration of the inhibitor. Top-down mass-spectrometric analysis shows that the main binding of CLR01 to α-synuclein occurs at the N-terminal Lys-10/Lys-12. Photo-induced cross-linking of unmodified proteins (PICUP) analysis shows that under the conditions used for the fluorescence analysis, α-synuclein is predominantly monomeric. The results can be successfully modeled using a kinetic scheme in which two aggregation-prone monomers can form an encounter complex that leads to further oligomerization but can also dissociate back to monomers if the reconfiguration rate is sufficiently high. Taken together, the data provide important insights into the preferred binding site of CLR01 on α-synuclein and the mechanism by which the molecular tweezer prevents self-assembly into neurotoxic aggregates by α-synuclein and presumably other amyloidogenic proteins.
Keywords: Inhibitor; Intramolecular Diffusion; Mass Spectrometry (MS); Parkinson's Disease; Protein Aggregation; Spectroscopy; α-Synuclein.
Figures







Similar articles
-
Characterization of Molecular Tweezer Binding on α-Synuclein with Native Top-Down Mass Spectrometry and Ion Mobility-Mass Spectrometry Reveals a Mechanism for Aggregation Inhibition.J Am Soc Mass Spectrom. 2023 Dec 6;34(12):2739-2747. doi: 10.1021/jasms.3c00281. Epub 2023 Nov 7. J Am Soc Mass Spectrom. 2023. PMID: 37936057 Free PMC article.
-
CLR01 protects dopaminergic neurons in vitro and in mouse models of Parkinson's disease.Nat Commun. 2020 Sep 28;11(1):4885. doi: 10.1038/s41467-020-18689-x. Nat Commun. 2020. PMID: 32985503 Free PMC article.
-
Native Top-Down Mass Spectrometry and Ion Mobility Spectrometry of the Interaction of Tau Protein with a Molecular Tweezer Assembly Modulator.J Am Soc Mass Spectrom. 2019 Jan;30(1):16-23. doi: 10.1007/s13361-018-2027-6. Epub 2018 Jul 30. J Am Soc Mass Spectrom. 2019. PMID: 30062477 Free PMC article.
-
Alteration of Structure and Aggregation of α-Synuclein by Familial Parkinson's Disease Associated Mutations.Curr Protein Pept Sci. 2017;18(7):656-676. doi: 10.2174/1389203717666160314151706. Curr Protein Pept Sci. 2017. PMID: 26972727 Review.
-
The aggregation and fibrillation of alpha-synuclein.Acc Chem Res. 2006 Sep;39(9):628-34. doi: 10.1021/ar050073t. Acc Chem Res. 2006. PMID: 16981679 Review.
Cited by
-
Folding and self-assembly of short intrinsically disordered peptides and protein regions.Nanoscale Adv. 2021 Jan 18;3(7):1789-1812. doi: 10.1039/d0na00941e. eCollection 2021 Apr 6. Nanoscale Adv. 2021. PMID: 36133101 Free PMC article. Review.
-
Neurotoxicity of the Parkinson Disease-Associated Pesticide Ziram Is Synuclein-Dependent in Zebrafish Embryos.Environ Health Perspect. 2016 Nov;124(11):1766-1775. doi: 10.1289/EHP141. Epub 2016 Jun 15. Environ Health Perspect. 2016. PMID: 27301718 Free PMC article.
-
Effects of Mutations on the Reconfiguration Rate of α-Synuclein.J Phys Chem B. 2015 Dec 17;119(50):15443-50. doi: 10.1021/acs.jpcb.5b10136. Epub 2015 Dec 4. J Phys Chem B. 2015. PMID: 26572968 Free PMC article.
-
Inhibition of Mutant αB Crystallin-Induced Protein Aggregation by a Molecular Tweezer.J Am Heart Assoc. 2017 Aug 8;6(8):e006182. doi: 10.1161/JAHA.117.006182. J Am Heart Assoc. 2017. PMID: 28862927 Free PMC article.
-
Protein aggregation and therapeutic strategies in SOD1- and TDP-43- linked ALS.Front Mol Biosci. 2024 May 24;11:1383453. doi: 10.3389/fmolb.2024.1383453. eCollection 2024. Front Mol Biosci. 2024. PMID: 38855322 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

