MarvelD3 couples tight junctions to the MEKK1-JNK pathway to regulate cell behavior and survival
- PMID: 24567356
- PMCID: PMC3941049
- DOI: 10.1083/jcb.201304115
MarvelD3 couples tight junctions to the MEKK1-JNK pathway to regulate cell behavior and survival
Abstract
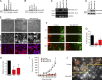
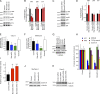
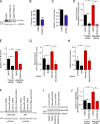
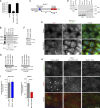
MarvelD3 is a transmembrane component of tight junctions, but there is little evidence for a direct involvement in the junctional permeability barrier. Tight junctions also regulate signaling mechanisms that guide cell proliferation; however, the transmembrane components that link the junction to such signaling pathways are not well understood. In this paper, we show that MarvelD3 is a dynamic junctional regulator of the MEKK1-c-Jun NH2-terminal kinase (JNK) pathway. Loss of MarvelD3 expression in differentiating Caco-2 cells resulted in increased cell migration and proliferation, whereas reexpression in a metastatic tumor cell line inhibited migration, proliferation, and in vivo tumor formation. Expression levels of MarvelD3 inversely correlated with JNK activity, as MarvelD3 recruited MEKK1 to junctions, leading to down-regulation of JNK phosphorylation and inhibition of JNK-regulated transcriptional mechanisms. Interplay between MarvelD3 internalization and JNK activation tuned activation of MEKK1 during osmotic stress, leading to junction dissociation and cell death in MarvelD3-depleted cells. MarvelD3 thus couples tight junctions to the MEKK1-JNK pathway to regulate cell behavior and survival.
Figures

References
-
- Antonetti D.A., Barber A.J., Khin S., Lieth E., Tarbell J.M., Gardner T.W., and Penn State Retina Research Group 1998. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 47:1953–1959 10.2337/diabetes.47.12.1953 - DOI - PubMed
-
- Antonetti D.A., Barber A.J., Hollinger L.A., Wolpert E.B., Gardner T.W. 1999. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 274:23463–23467 10.1074/jbc.274.33.23463 - DOI - PubMed
-
- Balda M.S., Whitney J.A., Flores C., González S., Cereijido M., Matter K. 1996. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134:1031–1049 10.1083/jcb.134.4.1031 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

