Caveolae regulate the nanoscale organization of the plasma membrane to remotely control Ras signaling
- PMID: 24567358
- PMCID: PMC3941050
- DOI: 10.1083/jcb.201307055
Caveolae regulate the nanoscale organization of the plasma membrane to remotely control Ras signaling
Abstract
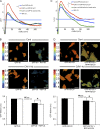
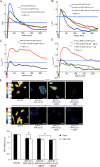
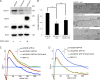
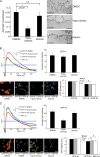
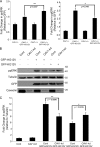
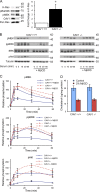
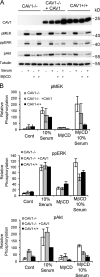
The molecular mechanisms whereby caveolae exert control over cellular signaling have to date remained elusive. We have therefore explored the role caveolae play in modulating Ras signaling. Lipidomic and gene array analyses revealed that caveolin-1 (CAV1) deficiency results in altered cellular lipid composition, and plasma membrane (PM) phosphatidylserine distribution. These changes correlated with increased K-Ras expression and extensive isoform-specific perturbation of Ras spatial organization: in CAV1-deficient cells K-RasG12V nanoclustering and MAPK activation were enhanced, whereas GTP-dependent lateral segregation of H-Ras was abolished resulting in compromised signal output from H-RasG12V nanoclusters. These changes in Ras nanoclustering were phenocopied by the down-regulation of Cavin1, another crucial caveolar structural component, and by acute loss of caveolae in response to increased osmotic pressure. Thus, we postulate that caveolae remotely regulate Ras nanoclustering and signal transduction by controlling PM organization. Similarly, caveolae transduce mechanical stress into PM lipid alterations that, in turn, modulate Ras PM organization.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

