Clinical targeting of mutated and wild-type protein tyrosine kinases in cancer
- PMID: 24567371
- PMCID: PMC4019040
- DOI: 10.1128/MCB.01592-13
Clinical targeting of mutated and wild-type protein tyrosine kinases in cancer
Abstract
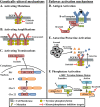
Clinical therapies for cancer have evolved from toxic, nontargeted agents to manageable, highly targeted therapies. Protein tyrosine kinases are a family of signaling molecules implicated in nearly every cancer type and are the foundation for the development of modern targeted agents. Recent genomic analyses have identified activating mutations, translocations, and amplifications of tyrosine kinases. Selective targeting of these genetically altered tyrosine kinases has resulted in significant clinical advances, including increased patient survival. This indicates that altered protein tyrosine kinases are the main drivers of many different cancers. However, lost during analyses of genetic lesions are the contributions of activated, wild-type kinases on tumor-dependent pathways. New approaches in phosphoproteomic technologies have identified several wild-type tyrosine kinase activation states, suggesting that non-genetically altered kinases can be essential "nodes" for signal transduction. Here, we summarize the evidence supporting the common mechanisms of protein tyrosine kinase activation in cancer and provide a personal perspective on the kinases BCR-ABL and BTK, as well as nonmutated kinase targets in prostate cancer, through our work. We outline the mechanisms of tyrosine kinase activation in the absence of direct mutation and discuss whether non-genetically altered tyrosine kinases or their associated downstream signaling pathways can be effectively targeted.
Figures
References
-
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. 2001. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344:1038–1042. 10.1056/NEJM200104053441402 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
