Toll-like receptor 10 is involved in induction of innate immune responses to influenza virus infection
- PMID: 24567377
- PMCID: PMC3956146
- DOI: 10.1073/pnas.1324266111
Toll-like receptor 10 is involved in induction of innate immune responses to influenza virus infection
Abstract
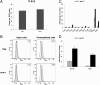
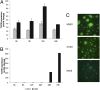
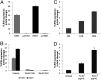
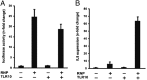
Toll-like receptors (TLRs) play key roles in innate immune recognition of pathogen-associated molecular patterns of invading microbes. Among the 10 TLR family members identified in humans, TLR10 remains an orphan receptor without known agonist or function. TLR10 is a pseudogene in mice and mouse models are noninformative in this regard. Using influenza virus infection in primary human peripheral blood monocyte-derived macrophages and a human monocytic cell line, we now provide previously unidentified evidence that TLR10 plays a role in innate immune responses following viral infection. Influenza virus infection increased TLR10 expression and TLR10 contributed to innate immune sensing of viral infection leading to cytokine induction, including proinflammatory cytokines and interferons. TLR10 induction is more pronounced following infection with highly pathogenic avian influenza H5N1 virus compared with a low pathogenic H1N1 virus. Induction of TLR10 by virus infection requires active virus replication and de novo protein synthesis. Culture supernatants of virus-infected cells modestly up-regulate TLR10 expression in nonvirus-infected cells. Signaling via TLR10 was activated by the functional RNA-protein complex of influenza virus leading to robust induction of cytokine expression. Taken together, our findings identify TLR10 as an important innate immune sensor of viral infection and its role in innate immune defense and immunopathology following viral and bacterial pathogens deserves attention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. - PubMed
-
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. - PubMed
-
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. - PubMed
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

