Cisplatin, 5-fluorouracil, and cetuximab (PFE) with or without cilengitide in recurrent/metastatic squamous cell carcinoma of the head and neck: results of the randomized phase I/II ADVANTAGE trial (phase II part)
- PMID: 24567516
- PMCID: PMC3933250
- DOI: 10.1093/annonc/mdu003
Cisplatin, 5-fluorouracil, and cetuximab (PFE) with or without cilengitide in recurrent/metastatic squamous cell carcinoma of the head and neck: results of the randomized phase I/II ADVANTAGE trial (phase II part)
Abstract
Background: Recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M-SCCHN) overexpresses αvβ5 integrin. Cilengitide selectively inhibits αvβ3 and αvβ5 integrins and is investigated as a treatment strategy.
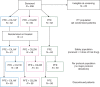
Patients and methods: The phase I/II study ADVANTAGE evaluated cilengitide combined with cisplatin, 5-fluorouracil, and cetuximab (PFE) in R/M-SCCHN. The phase II part reported here was an open-label, randomized, controlled trial investigating progression-free survival (PFS). Patients received up to six cycles of PFE alone or combined with cilengitide 2000 mg once (CIL1W) or twice (CIL2W) weekly. Thereafter, patients received maintenance therapy (cilengitide arms: cilengitide plus cetuximab; PFE-alone arm: cetuximab only) until disease progression or unacceptable toxicity.
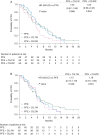
Results: One hundred and eighty-two patients were treated. Median PFS per investigator read was similar for CIL1W + PFE, CIL2W + PFE, and PFE alone (6.4, 5.6, and 5.7 months, respectively). Accordingly, median overall survival and objective response rates were not improved with cilengitide (12.4 months/47%, 10.6 months/27%, and 11.6 months/36%, respectively). No clinically meaningful safety differences were observed between groups. None of the tested biomarkers (expression of integrins, CD31, Ki-67, vascular endothelial growth factor receptor 2, vascular endothelial-cadherin, type IV collagen, epidermal growth factor receptor, or p16 for human papillomavirus) were predictive of outcome.
Conclusion: Neither of the cilengitide-containing regimens demonstrated a PFS benefit over PFE alone in R/M-SCCHN patients.
Keywords: cetuximab; cilengitide; integrin inhibitor; phase I/II; platinum-based chemotherapy with 5-fluorouracil; recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M-SCCHN).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

