Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity
- PMID: 24567531
- PMCID: PMC3965631
- DOI: 10.4049/jimmunol.1302985
Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity
Abstract
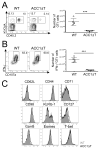
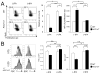
Fatty acids (FAs) are essential constituents of cell membranes, signaling molecules, and bioenergetic substrates. Because CD8(+) T cells undergo both functional and metabolic changes during activation and differentiation, dynamic changes in FA metabolism also occur. However, the contributions of de novo lipogenesis to acquisition and maintenance of CD8(+) T cell function are unclear. In this article, we demonstrate the role of FA synthesis in CD8(+) T cell immunity. T cell-specific deletion of acetyl coenzyme A carboxylase 1 (ACC1), an enzyme that catalyzes conversion of acetyl coenzyme A to malonyl coenzyme A, a carbon donor for long-chain FA synthesis, resulted in impaired peripheral persistence and homeostatic proliferation of CD8(+) T cells in naive mice. Loss of ACC1 did not compromise effector CD8(+) T cell differentiation upon listeria infection but did result in a severe defect in Ag-specific CD8(+) T cell accumulation because of increased death of proliferating cells. Furthermore, in vitro mitogenic stimulation demonstrated that defective blasting and survival of ACC1-deficient CD8(+) T cells could be rescued by provision of exogenous FA. These results suggest an essential role for ACC1-mediated de novo lipogenesis as a regulator of CD8(+) T cell expansion, and may provide insights for therapeutic targets for interventions in autoimmune diseases, cancer, and chronic infections.
Figures




References
-
- van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. - PubMed
-
- Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. - PubMed
-
- Drake DR, 3rd, Braciale TJ. Cutting edge: lipid raft integrity affects the efficiency of MHC class I tetramer binding and cell surface TCR arrangement on CD8+ T cells. J Immunol. 2001;166:7009–7013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

