Impact of 13-Valent Pneumococcal Conjugate Vaccination on Streptococcus pneumoniae Carriage in Young Children in Massachusetts
- PMID: 24567842
- PMCID: PMC3933044
- DOI: 10.1093/jpids/pit057
Impact of 13-Valent Pneumococcal Conjugate Vaccination on Streptococcus pneumoniae Carriage in Young Children in Massachusetts
Abstract
Background: In April 2010, a 13-valent pneumococcal conjugate vaccine (PCV13) replaced PCV7 for use in the United States. We evaluated rates of pneumococcal colonization, by serotype and antibiotic resistance, in Massachusetts communities where serial cross-sectional surveillance has been conducted for the past decade.
Methods: Nasopharyngeal swabs were obtained from children 0 to <7 years of age and seen by primary care providers for well child or acute illness visits in 2001, 2004, 2007, 2009, and 2011. Pneumococcal isolates were serotyped by Quellung reaction and classified as PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, 23F), additional PCV13 serotypes (1, 3, 5, 6A, 7F, 19A), or non-PCV13 serotypes. Changes in colonization and impact of PCV13 were assessed using generalized linear mixed models, adjusting for known risk factors and accounting for clustering by community.
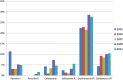
Results: Introduction of PCV13 did not affect the rate of overall pneumococcal colonization (31% in 2011). Colonization with non-PCV13 serotypes increased between 2001 and 2011 for all children (odds ratio [OR] per year, 1.12; 95% confidence interval [CI], 1.10, 1.15; P < .0001). 19A remained the second most common serotype in 2011, although a decline from 2009 was observed. Penicillin (7%), erythromycin (28%), ceftriaxone (10%), and clindamycin (10%) nonsusceptibility were commonly identified, concentrated among a small number of serotypes (including 19A, 35B, 15B/C, and 15A). Among healthy children 6-23 months old, colonization with PCV13 serotypes was lower among recipients of PCV13 vaccine (adjusted OR, 0.30; 95% CI, 0.11, 0.78). This effect was not observed in 6- to 23-month-old children with a concomitant respiratory tract infection (adjusted OR 1.36; 95% CI, 0.66, 2.77) or children 2 to <7 years old (adjusted OR, 1.17; 95% CI, 0.58, 2.34).
Conclusions: 13-Valent pneumococcal conjugate vaccine reduced the prevalence of colonization with PCV13 serotypes among children 6-23 months old, but its efficacy was not shown among older children.
Keywords: Streptococcus pneumoniae; colonization; pneumococcal conjugate vaccine.
© The Author 2013. Published by Oxford University Press on behalf of the Pediatric Infectious Diseases Society. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Centers for Disease Control and Prevention (CDC) Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine - United States, 2007. MMWR Morb Mortal Wkly Rep. 2010;59:253–7. - PubMed
-
- O'Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–20. - PubMed
-
- Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468–72. - PubMed
-
- Sharma D, Baughman W, Holst A, et al. Pneumococcal carriage and invasive disease in children before introduction of the 13-valent conjugate vaccine: comparison with the pre-7-valent conjugate vaccine era. Pediatr Infect Dis J. 2013;32 e45–53. - PubMed
-
- Weatherholtz R, Millar EV, Moulton LH, et al. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis. 2010;50:1238–46. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

