Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock
- PMID: 24567902
- PMCID: PMC3929910
- DOI: 10.1016/j.molmet.2013.10.005
Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock
Erratum in
-
Erratum to "Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock" [Mol Metab 3 (2014) 29-41].Mol Metab. 2014 Sep 16;3(9):857. doi: 10.1016/j.molmet.2014.09.002. eCollection 2014 Dec. Mol Metab. 2014. PMID: 25503566 Free PMC article.
Abstract
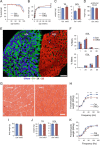
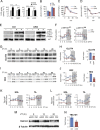
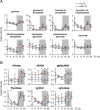
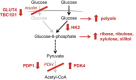
Circadian rhythms control metabolism and energy homeostasis, but the role of the skeletal muscle clock has never been explored. We generated conditional and inducible mouse lines with muscle-specific ablation of the core clock gene Bmal1. Skeletal muscles from these mice showed impaired insulin-stimulated glucose uptake with reduced protein levels of GLUT4, the insulin-dependent glucose transporter, and TBC1D1, a Rab-GTPase involved in GLUT4 translocation. Pyruvate dehydrogenase (PDH) activity was also reduced due to altered expression of circadian genes Pdk4 and Pdp1, coding for PDH kinase and phosphatase, respectively. PDH inhibition leads to reduced glucose oxidation and diversion of glycolytic intermediates to alternative metabolic pathways, as revealed by metabolome analysis. The impaired glucose metabolism induced by muscle-specific Bmal1 knockout suggests that a major physiological role of the muscle clock is to prepare for the transition from the rest/fasting phase to the active/feeding phase, when glucose becomes the predominant fuel for skeletal muscle.
Keywords: 2-DG, 2-Deoxyglucose; BSA, bovine serum albumin; Bmal1; Circadian rhythms; GSEA, Gene Set Enrichment Analysis; Glucose metabolism; Glucose uptake; HK2, hexokinase 2; KHB, Krebs–Henseleit buffer; Muscle insulin resistance; PDH, pyruvate dehydrogenase; PDK, PDH kinase; PDP, PDH phosphatase; SCN, suprachiasmatic nucleus; Skeletal muscle; ZT, Zeitgeber time; imKO, inducible muscle-specific Bmal1 knockout; mKO, muscle-specific Bmal1 knockout.
Figures


References
-
- Asher G., Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metabolism. 2011;13:125–137. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

