The reward value of sucrose in leptin-deficient obese mice
- PMID: 24567906
- PMCID: PMC3929919
- DOI: 10.1016/j.molmet.2013.10.007
The reward value of sucrose in leptin-deficient obese mice
Abstract
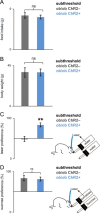
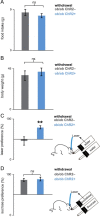
Leptin-deficient patients report higher "liking" ratings for food, and leptin replacement therapy normalizes these ratings even before weight loss is achieved. Since animals cannot report their ratings, we studied the relationship between leptin and food reward in leptin-deficient ob/ob mice using a optogenetic assay that quantifies the reward value of sucrose. In this assay, mice chose between one sipper dispensing the artificial sweetener sucralose coupled to optogenetic activation of dopaminergic (DA) neurons, and another sipper dispensing sucrose. We found that the reward value of sucrose was high under a state of leptin deficiency, as well as at a dose of leptin that does not suppress food intake (12.5 ng/h). Treatment with higher doses of leptin decreased the reward value of sucrose before weight loss was achieved (100 ng/h), as seen in leptin-deficient patients. These results phenocopy in mice the behavior of leptin-deficient patients.
Keywords: Food preference; Leptin; Obesity; Optogenetics; Reward value; Sucrose.
Figures




References
-
- Friedman J.M. Modern science versus the stigma of obesity. Nature Medicine. 2004;10:563–569. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

