CD4 T cell activation by B cells in human Leishmania (Viannia) infection
- PMID: 24568275
- PMCID: PMC3937821
- DOI: 10.1186/1471-2334-14-108
CD4 T cell activation by B cells in human Leishmania (Viannia) infection
Abstract
Background: An effective adaptive immune response requires activation of specific CD4 T cells. The capacity of B cells to activate CD4 T cells in human cutaneous leishmaniasis caused by Leishmania (Viannia) has not been evaluated.
Methods: CD4 T cell activation by B cells of cutaneous leishmaniasis patients was evaluated by culture of PBMCs or purified B cells and CD4 T cells with Leishmania panamensis antigens. CD4 T cell and B cell activation markers were evaluated by flow cytometry and 13 cytokines were measured in supernatants with a bead-based capture assay. The effect of Leishmania antigens on BCR-mediated endocytosis of ovalbumin was evaluated in the Ramos human B cell line by targeting the antigen with anti-IgM-biotin and anti-biotin-ovalbumin-FITC.
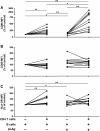
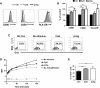
Results: Culture of PBMCs from cutaneous leishmaniasis patients with Leishmania antigens resulted in upregulation of the activation markers CD25 and CD69 as well as increased frequency of CD25hiCD127- cells among CD4 T cells. Concomitantly, B cells upregulated the costimulatory molecule CD86. These changes were not observed in PBMCs from healthy subjects, indicating participation of Leishmania-specific lymphocytes expanded in vivo. Purified B cells from these patients, when interacting with purified CD4 T cells and Leishmania antigens, were capable of inducing significant increases in CD25 and CD69 expression and CD25hiCD127- frequency in CD4 T cells. These changes were associated with upregulation of CD86 in B cells. Comparison of changes in CD4 T cell activation parameters between PBMC and B cell/CD4 T cell cultures showed no statistically significant differences; further, significant secretion of IFN-γ, TNF-α, IL-6 and IL-13 was induced in both types of cultures. Additionally, culture with Leishmania antigens enhanced BCR-mediated endocytosis of ovalbumin in Ramos human B cells.
Conclusions: The capacity of B cells specific for Leishmania antigens in peripheral blood of cutaneous leishmaniasis patients to activate CD4 T cells and induce cytokine secretion is similar to that of all cell populations present in PBMCs. This capacity implicates B cells as a plausible target for modulation of the immune response to Leishmania infection as a therapeutic strategy.
Figures





Similar articles
-
T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure.Exp Parasitol. 1996 Nov;84(2):144-55. doi: 10.1006/expr.1996.0100. Exp Parasitol. 1996. PMID: 8932764
-
CD4(+) T cells defined by their Vβ T cell receptor expression are associated with immunoregulatory profiles and lesion size in human leishmaniasis.Clin Exp Immunol. 2011 Sep;165(3):338-51. doi: 10.1111/j.1365-2249.2011.04430.x. Epub 2011 Jul 4. Clin Exp Immunol. 2011. PMID: 21726211 Free PMC article.
-
Immunopathological characterization of human cutaneous leishmaniasis lesions caused by Leishmania (Viannia) spp. in Amazonian Brazil.Parasitol Res. 2017 May;116(5):1423-1431. doi: 10.1007/s00436-017-5403-4. Epub 2017 Feb 21. Parasitol Res. 2017. PMID: 28224222
-
Inflammasome Activation by CD8+ T Cells from Patients with Cutaneous Leishmaniasis Caused by Leishmania braziliensis in the Immunopathogenesis of the Disease.J Invest Dermatol. 2021 Jan;141(1):209-213.e2. doi: 10.1016/j.jid.2020.05.106. Epub 2020 Jun 13. J Invest Dermatol. 2021. PMID: 32544477 Review. No abstract available.
-
The Role of CD4 and CD8 T Cells in Human Cutaneous Leishmaniasis.Front Public Health. 2014 Sep 29;2:165. doi: 10.3389/fpubh.2014.00165. eCollection 2014. Front Public Health. 2014. PMID: 25325049 Free PMC article. Review.
Cited by
-
Coordinated roles for glycans in regulating the inhibitory function of CD22 on B cells.Biomed J. 2019 Aug;42(4):218-232. doi: 10.1016/j.bj.2019.07.010. Epub 2019 Sep 16. Biomed J. 2019. PMID: 31627864 Free PMC article. Review.
-
B-Cells and Antibodies as Contributors to Effector Immune Responses in Tuberculosis.Front Immunol. 2021 Feb 18;12:640168. doi: 10.3389/fimmu.2021.640168. eCollection 2021. Front Immunol. 2021. PMID: 33679802 Free PMC article. Review.
-
IL12p40 regulates functional development of human CD4+ T cells: enlightenment by the elevated expressions of IL12p40 in patients with inflammatory bowel diseases.Medicine (Baltimore). 2015 Mar;94(10):e613. doi: 10.1097/MD.0000000000000613. Medicine (Baltimore). 2015. PMID: 25761185 Free PMC article.
-
Applying Convergent Immunity to Innovative Vaccines Targeting Staphylococcus aureus.Front Immunol. 2014 Sep 26;5:463. doi: 10.3389/fimmu.2014.00463. eCollection 2014. Front Immunol. 2014. PMID: 25309545 Free PMC article. Review.
-
CINS: Cell Interaction Network inference from Single cell expression data.PLoS Comput Biol. 2022 Sep 12;18(9):e1010468. doi: 10.1371/journal.pcbi.1010468. eCollection 2022 Sep. PLoS Comput Biol. 2022. PMID: 36095011 Free PMC article.
References
-
- Bogdan C. Leishmaniasis in rheumatology, haematology and oncology: epidemiological, immunological and clinical aspects and caveats. Ann Rheum Dis. 2012;14(Suppl 2):i60–i66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

