Acquired defects in CFTR-dependent β-adrenergic sweat secretion in chronic obstructive pulmonary disease
- PMID: 24568560
- PMCID: PMC4015030
- DOI: 10.1186/1465-9921-15-25
Acquired defects in CFTR-dependent β-adrenergic sweat secretion in chronic obstructive pulmonary disease
Abstract
Rationale: Smoking-induced chronic obstructive pulmonary disease (COPD) is associated with acquired systemic cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. Recently, sweat evaporimetry has been shown to efficiently measure β-adrenergic sweat rate and specifically quantify CFTR function in the secretory coil of the sweat gland.
Objectives: To evaluate the presence and severity of systemic CFTR dysfunction in smoking-related lung disease using sweat evaporimetry to determine CFTR-dependent sweat rate.
Methods: We recruited a cohort of patients consisting of healthy never smokers (N = 18), healthy smokers (12), COPD smokers (25), and COPD former smokers (12) and measured β-adrenergic sweat secretion rate with evaporative water loss, sweat chloride, and clinical data (spirometry and symptom questionnaires).
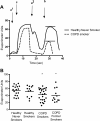
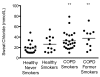
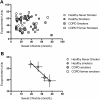
Measurements and main results: β-adrenergic sweat rate was reduced in COPD smokers (41.9 ± 3.4, P < 0.05, ± SEM) and COPD former smokers (39.0 ± 5.4, P < 0.05) compared to healthy controls (53.6 ± 3.4). Similarly, sweat chloride was significantly greater in COPD smokers (32.8 ± 3.3, P < 0.01) and COPD former smokers (37.8 ± 6.0, P < 0.01) vs. healthy controls (19.1 ± 2.5). Univariate analysis revealed a significant association between β-adrenergic sweat rate and female gender (β = 0.26), age (-0.28), FEV1% (0.35), dyspnea (-0.3), and history of smoking (-0.27; each P < 0.05). Stepwise multivariate regression included gender (0.39) and COPD (-0.43) in the final model (R()2 = 0.266, P < 0.0001).
Conclusions: β-adrenergic sweat rate was significantly reduced in COPD patients, regardless of smoking status, reflecting acquired CFTR dysfunction and abnormal gland secretion in the skin that can persist despite smoking cessation. β-adrenergic sweat rate and sweat chloride are associated with COPD severity and clinical symptoms, supporting the hypothesis that CFTR decrements have a causative role in COPD pathogenesis.
Figures

Similar articles
-
Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function.Am J Respir Crit Care Med. 2013 Dec 1;188(11):1321-30. doi: 10.1164/rccm.201304-0733OC. Am J Respir Crit Care Med. 2013. PMID: 24040746 Free PMC article.
-
Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD.Chest. 2013 Aug;144(2):498-506. doi: 10.1378/chest.13-0274. Chest. 2013. PMID: 23538783 Free PMC article.
-
β-adrenergic sweat secretion as a diagnostic test for cystic fibrosis.Am J Respir Crit Care Med. 2012 Oct 15;186(8):732-9. doi: 10.1164/rccm.201205-0922OC. Epub 2012 Aug 2. Am J Respir Crit Care Med. 2012. PMID: 22859523
-
Cystic fibrosis transmembrane conductance regulator in COPD: a role in respiratory epithelium and beyond.Eur Respir J. 2023 Apr 1;61(4):2201307. doi: 10.1183/13993003.01307-2022. Print 2023 Apr. Eur Respir J. 2023. PMID: 37003609 Free PMC article. Review.
-
Cigarette Smoke-Induced Acquired Dysfunction of Cystic Fibrosis Transmembrane Conductance Regulator in the Pathogenesis of Chronic Obstructive Pulmonary Disease.Oxid Med Cell Longev. 2018 Apr 23;2018:6567578. doi: 10.1155/2018/6567578. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29849907 Free PMC article. Review.
Cited by
-
Therapeutic Approaches to Acquired Cystic Fibrosis Transmembrane Conductance Regulator Dysfunction in Chronic Bronchitis.Ann Am Thorac Soc. 2016 Apr;13 Suppl 2(Suppl 2):S169-76. doi: 10.1513/AnnalsATS.201509-601KV. Ann Am Thorac Soc. 2016. PMID: 27115953 Free PMC article. Review.
-
Sweat rate analysis of ivacaftor potentiation of CFTR in non-CF adults.Sci Rep. 2018 Nov 2;8(1):16233. doi: 10.1038/s41598-018-34308-8. Sci Rep. 2018. PMID: 30389955 Free PMC article. Clinical Trial.
-
Pilot evaluation of ivacaftor for chronic bronchitis.Lancet Respir Med. 2016 Jun;4(6):e32-3. doi: 10.1016/S2213-2600(16)30047-9. Epub 2016 May 16. Lancet Respir Med. 2016. PMID: 27185048 Free PMC article. Clinical Trial. No abstract available.
-
Vaporized E-Cigarette Liquids Induce Ion Transport Dysfunction in Airway Epithelia.Am J Respir Cell Mol Biol. 2019 Aug;61(2):162-173. doi: 10.1165/rcmb.2017-0432OC. Am J Respir Cell Mol Biol. 2019. PMID: 30576219 Free PMC article.
-
Dysfunction in the Cystic Fibrosis Transmembrane Regulator in Chronic Obstructive Pulmonary Disease as a Potential Target for Personalised Medicine.Biomedicines. 2021 Oct 10;9(10):1437. doi: 10.3390/biomedicines9101437. Biomedicines. 2021. PMID: 34680554 Free PMC article. Review.
References
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

