Crowning: a novel Escherichia coli colonizing behaviour generating a self-organized corona
- PMID: 24568619
- PMCID: PMC3936827
- DOI: 10.1186/1756-0500-7-108
Crowning: a novel Escherichia coli colonizing behaviour generating a self-organized corona
Abstract
Background: Encased in a matrix of extracellular polymeric substances (EPS) composed of flagella, adhesins, amyloid fibers (curli), and exopolysaccharides (cellulose, β-1,6-N-acetyl-D-glucosamine polymer-PGA-, colanic acid), the bacteria Escherichia coli is able to attach to and colonize different types of biotic and abiotic surfaces forming biofilms and colonies of intricate morphological architectures. Many of the biological aspects that underlie the generation and development of these E. coli's formations are largely poorly understood.
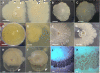
Results: Here, we report the characterization of a novel E. coli sessile behaviour termed "crowning" due to the bacterial generation of a new 3-D architectural pattern: a corona. This bacterial pattern is formed by joining bush-like multilayered "coronal flares or spikes" arranged in a ring, which self-organize through the growth, self-clumping and massive self-aggregation of cells tightly interacting inside semisolid agar on plastic surfaces. Remarkably, the corona's formation is developed independently of the adhesiveness of the major components of E. coli's EPS matrix, the function of chemotaxis sensory system, type 1 pili and the biofilm master regulator CsgD, but its formation is suppressed by flagella-driven motility and glucose. Intriguingly, this glucose effect on the corona development is not mediated by the classical catabolic repression system, the cyclic AMP (cAMP)-cAMP receptor protein (CRP) complex. Thus, corona formation departs from the canonical regulatory transcriptional core that controls biofilm formation in E. coli.
Conclusions: With this novel "crowning" activity, E. coli expands its repertoire of colonizing collective behaviours to explore, invade and exploit environments whose critical viscosities impede flagella driven-motility.
Figures


References
-
- Shapiro JA. In: Bacteria as Multicellular Organisms. Shapiro JA, Dworkin M, editor. New York: Oxford University Press; 1997. Multicellularity: the Rule not the Exception. Lessons from Escherichia Coli Colonies; pp. 14–49.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

