The G protein-coupled receptor GALR2 promotes angiogenesis in head and neck cancer
- PMID: 24568968
- PMCID: PMC4023835
- DOI: 10.1158/1535-7163.MCT-13-0904
The G protein-coupled receptor GALR2 promotes angiogenesis in head and neck cancer
Abstract
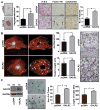
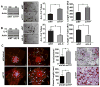
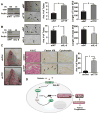
Squamous cell carcinoma of the head and neck (SCCHN) is an aggressive disease with poor patient survival. Galanin receptor 2 (GALR2) is a G protein-coupled receptor that induces aggressive tumor growth in SCCHN. The objective of this study was to investigate the mechanism by which GALR2 promotes angiogenesis, a critical oncogenic phenotype required for tumor growth. The impact of GALR2 expression on secretion of proangiogenic cytokines in multiple SCCHN cell lines was investigated by ELISA and in vitro angiogenesis assays. Chemical inhibitor and genetic knockdown strategies were used to understand the key regulators. The in vivo impact of GALR2 on angiogenesis was investigated in mouse xenograft, chick chorioallantoic membrane, and the clinically relevant mouse orthotopic floor-of-mouth models. GALR2 induced angiogenesis via p38-MAPK-mediated secretion of proangiogenic cytokines, VEGF, and interleukin-6 (IL-6). Moreover, GALR2 activated small-GTP-protein, RAP1B, thereby inducing p38-mediated inactivation of tristetraprolin (TTP), which functions to destabilize cytokine transcripts. This resulted in enhanced secretion of proangiogenic cytokines and angiogenesis in vitro and in vivo. In SCCHN cells overexpressing GALR2, inactivation of TTP increased secretion of IL-6 and VEGF, whereas inhibition of p38 activated TTP and decreased cytokine secretion. Here, we report that GALR2 stimulates tumor angiogenesis in SCCHN via p38-mediated inhibition of TTP with resultant enhanced cytokine secretion. Given that p38 inhibitors are in clinical use for inflammatory disorders, GALR2/p38-mediated cytokine secretion may be an excellent target for new adjuvant therapy in SCCHN.
Conflict of interest statement
Figures


Similar articles
-
Rap1 mediates galanin receptor 2-induced proliferation and survival in squamous cell carcinoma.Cell Signal. 2011 Jul;23(7):1110-8. doi: 10.1016/j.cellsig.2011.02.002. Epub 2011 Feb 21. Cell Signal. 2011. PMID: 21345369 Free PMC article.
-
Inactivation or loss of TTP promotes invasion in head and neck cancer via transcript stabilization and secretion of MMP9, MMP2, and IL-6.Clin Cancer Res. 2013 Mar 1;19(5):1169-79. doi: 10.1158/1078-0432.CCR-12-2927. Epub 2013 Jan 24. Clin Cancer Res. 2013. PMID: 23349315 Free PMC article.
-
The Proton-Sensing G-Protein Coupled Receptor GPR4 Promotes Angiogenesis in Head and Neck Cancer.PLoS One. 2016 Apr 14;11(4):e0152789. doi: 10.1371/journal.pone.0152789. eCollection 2016. PLoS One. 2016. PMID: 27078157 Free PMC article.
-
Palmitate differentially regulates Spexin, and its receptors Galr2 and Galr3, in GnRH neurons through mechanisms involving PKC, MAPKs, and TLR4.Mol Cell Endocrinol. 2020 Dec 1;518:110991. doi: 10.1016/j.mce.2020.110991. Epub 2020 Aug 22. Mol Cell Endocrinol. 2020. PMID: 32841709 Review.
-
Galanin receptor subtypes 1 and 2 as therapeutic targets in head and neck squamous cell carcinoma.Expert Opin Ther Targets. 2010 Mar;14(3):289-302. doi: 10.1517/14728221003598922. Expert Opin Ther Targets. 2010. PMID: 20148716 Review.
Cited by
-
Roles of Tristetraprolin in Tumorigenesis.Int J Mol Sci. 2018 Oct 29;19(11):3384. doi: 10.3390/ijms19113384. Int J Mol Sci. 2018. PMID: 30380668 Free PMC article. Review.
-
Galanin mediates tumor-induced immunosuppression in head and neck squamous cell carcinoma.Cell Oncol (Dordr). 2022 Apr;45(2):241-256. doi: 10.1007/s13402-021-00631-y. Epub 2022 Mar 10. Cell Oncol (Dordr). 2022. PMID: 35267186 Free PMC article.
-
Tristetraprolin, a Potential Safeguard Against Carcinoma: Role in the Tumor Microenvironment.Front Oncol. 2021 May 7;11:632189. doi: 10.3389/fonc.2021.632189. eCollection 2021. Front Oncol. 2021. PMID: 34026612 Free PMC article. Review.
-
Galanin modulates the neural niche to favour perineural invasion in head and neck cancer.Nat Commun. 2015 Apr 28;6:6885. doi: 10.1038/ncomms7885. Nat Commun. 2015. PMID: 25917569 Free PMC article.
-
Cancer neuroscience in head and neck: interactions, modulation, and therapeutic strategies.Mol Cancer. 2025 Mar 31;24(1):101. doi: 10.1186/s12943-025-02299-6. Mol Cancer. 2025. PMID: 40165230 Free PMC article. Review.
References
-
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. - PubMed
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Lingen MW. Angiogenesis in the development of head and neck cancer and its inhibition by chemopreventive agents. Crit Rev Oral Biol Med. 1999;10:153–64. - PubMed
-
- Pries R, Nitsch S, Wollenberg B. Role of cytokines in head and neck squamous cell carcinoma. Expert review of anticancer therapy. 2006;6:1195–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

