Small-molecule activator of glutamate transporter EAAT2 translation provides neuroprotection
- PMID: 24569372
- PMCID: PMC3938250
- DOI: 10.1172/JCI66163
Small-molecule activator of glutamate transporter EAAT2 translation provides neuroprotection
Abstract
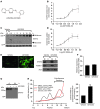
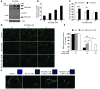
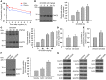
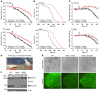
Glial glutamate transporter EAAT2 plays a major role in glutamate clearance in synaptic clefts. Several lines of evidence indicate that strategies designed to increase EAAT2 expression have potential for preventing excitotoxicity, which contributes to neuronal injury and death in neurodegenerative diseases. We previously discovered several classes of compounds that can increase EAAT2 expression through translational activation. Here, we present efficacy studies of the compound LDN/OSU-0212320, which is a pyridazine derivative from one of our lead series. In a murine model, LDN/OSU-0212320 had good potency, adequate pharmacokinetic properties, no observed toxicity at the doses examined, and low side effect/toxicity potential. Additionally, LDN/OSU-0212320 protected cultured neurons from glutamate-mediated excitotoxic injury and death via EAAT2 activation. Importantly, LDN/OSU-0212320 markedly delayed motor function decline and extended lifespan in an animal model of amyotrophic lateral sclerosis (ALS). We also found that LDN/OSU-0212320 substantially reduced mortality, neuronal death, and spontaneous recurrent seizures in a pilocarpine-induced temporal lobe epilepsy model. Moreover, our study demonstrated that LDN/OSU-0212320 treatment results in activation of PKC and subsequent Y-box-binding protein 1 (YB-1) activation, which regulates activation of EAAT2 translation. Our data indicate that the use of small molecules to enhance EAAT2 translation may be a therapeutic strategy for the treatment of neurodegenerative diseases.
Figures


Comment in
-
Translational enhancers of EAAT2: therapeutic implications for neurodegenerative disease.J Clin Invest. 2014 Mar;124(3):964-7. doi: 10.1172/JCI74608. Epub 2014 Feb 24. J Clin Invest. 2014. PMID: 24569369 Free PMC article.
References
-
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15(3):711–720. doi: 10.1016/0896-6273(95)90158-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

