Cell-specific translational profiling in acute kidney injury
- PMID: 24569379
- PMCID: PMC3938273
- DOI: 10.1172/JCI72126
Cell-specific translational profiling in acute kidney injury
Erratum in
- J Clin Invest. 2014 May 1;124(5):2288. Humphreys, Benjamin [corrected to Humphreys, Benjamin D]
Abstract
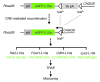
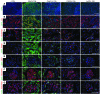
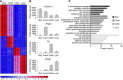
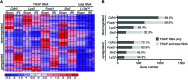
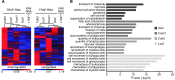
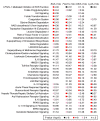
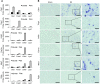
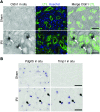
Acute kidney injury (AKI) promotes an abrupt loss of kidney function that results in substantial morbidity and mortality. Considerable effort has gone toward identification of diagnostic biomarkers and analysis of AKI-associated molecular events; however, most studies have adopted organ-wide approaches and have not elucidated the interplay among different cell types involved in AKI pathophysiology. To better characterize AKI-associated molecular and cellular events, we developed a mouse line that enables the identification of translational profiles in specific cell types. This strategy relies on CRE recombinase-dependent activation of an EGFP-tagged L10a ribosomal protein subunit, which allows translating ribosome affinity purification (TRAP) of mRNA populations in CRE-expressing cells. Combining this mouse line with cell type-specific CRE-driver lines, we identified distinct cellular responses in an ischemia reperfusion injury (IRI) model of AKI. Twenty-four hours following IRI, distinct translational signatures were identified in the nephron, kidney interstitial cell populations, vascular endothelium, and macrophages/monocytes. Furthermore, TRAP captured known IRI-associated markers, validating this approach. Biological function annotation, canonical pathway analysis, and in situ analysis of identified response genes provided insight into cell-specific injury signatures. Our study provides a deep, cell-based view of early injury-associated molecular events in AKI and documents a versatile, genetic tool to monitor cell-specific and temporal-specific biological processes in disease modeling.
Figures
References
-
- Shusterman N, Strom BL, Murray TG, Morrison G, West SL, Maislin G. Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am J Med. 1987;83(1):65–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

