Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states
- PMID: 24569463
- PMCID: PMC3960630
- DOI: 10.1038/bjc.2014.80
Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states
Abstract
Background: Eribulin mesilate (eribulin), a non-taxane microtubule dynamics inhibitor, has shown trends towards greater overall survival (OS) compared with progression-free survival in late-stage metastatic breast cancer patients in the clinic. This finding suggests that eribulin may have additional, previously unrecognised antitumour mechanisms beyond its established antimitotic activity. To investigate this possibility, eribulin's effects on the balance between epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET) in human breast cancer cells were investigated.
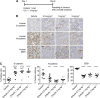
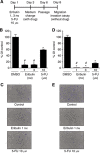
Methods: Triple negative breast cancer (TNBC) cells, which are oestrogen receptor (ER-)/progesterone receptor (PR-)/human epithelial growth receptor 2 (HER2-) and have a mesenchymal phenotype, were treated with eribulin for 7 days, followed by measurement of EMT-related gene and protein expression changes in the surviving cells by quantitative real-time PCR (qPCR) and immunoblot, respectively. In addition, proliferation, migration, and invasion assays were also conducted in eribulin-treated cells. To investigate the effects of eribulin on TGF-β/Smad signalling, the phosphorylation status of Smad proteins was analysed. In vivo, the EMT/MET status of TNBC xenografts in mice treated with eribulin was examined by qPCR, immunoblot, and immunohistochemical analysis. Finally, an experimental lung metastasis model was utilised to gauge the metastatic activity of eribulin-treated TNBC in the in vivo setting.
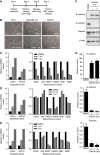
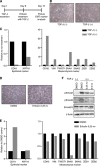
Results: Treatment of TNBC cells with eribulin in vitro led to morphological changes consistent with transition from a mesenchymal to an epithelial phenotype. Expression analyses of EMT markers showed that eribulin treatment led to decreased expression of several mesenchymal marker genes, together with increased expression of several epithelial markers. In the TGF-β induced EMT model, eribulin treatment reversed EMT, coincident with inhibition of Smad2 and Smad3 phosphorylation. Consistent with these changes, TNBC cells treated with eribulin for 7 days showed decreased capacity for in vitro migration and invasiveness. In in vivo xenograft models, eribulin treatment reversed EMT and induced MET as assessed by qPCR, immunoblot, and immunohistochemical analyses of epithelial and mesenchymal marker proteins. Finally, surviving TNBC cells pretreated in vitro with eribulin for 7 days led to decreased numbers of lung metastasis when assessed in an in vivo experimental metastasis model.
Conclusions: Eribulin exerted significant effects on EMT/MET-related pathway components in human breast cancer cells in vitro and in vivo, consistent with a phenotypic switch from mesenchymal to epithelial states, and corresponding to observed decreases in migration and invasiveness in vitro as well as experimental metastasis in vivo. These preclinical findings may provide a plausible scientific basis for clinical observations of prolonged OS by suppression of further spread of metastasis in breast cancer patients treated with eribulin.
Figures

References
-
- Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9 (1:16–32. - PubMed
-
- Bonnomet A, Syne L, Brysse A, Feyereisen E, Thompson EW, Noel A, Foidart JM, Birembaut P, Polette M, Gilles C. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene. 2012;31 (33:3741–3753. - PubMed
-
- Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13 (8:2329–2334. - PubMed
-
- Chung JH, Rho JK, Xu X, Lee JS, Yoon HI, Lee CT, Choi YJ, Kim HR, Kim CH, Lee JC. Clinical and molecular evidences of epithelial to mesenchymal transition in acquired resistance to EGFR-TKIs. Lung Cancer. 2011;73 (2:176–182. - PubMed
-
- Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C, investigators E. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377 (9769:914–923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

