The log-dynamic brain: how skewed distributions affect network operations
- PMID: 24569488
- PMCID: PMC4051294
- DOI: 10.1038/nrn3687
The log-dynamic brain: how skewed distributions affect network operations
Abstract
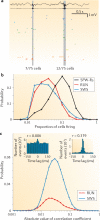
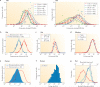
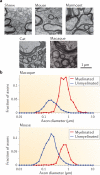
We often assume that the variables of functional and structural brain parameters - such as synaptic weights, the firing rates of individual neurons, the synchronous discharge of neural populations, the number of synaptic contacts between neurons and the size of dendritic boutons - have a bell-shaped distribution. However, at many physiological and anatomical levels in the brain, the distribution of numerous parameters is in fact strongly skewed with a heavy tail, suggesting that skewed (typically lognormal) distributions are fundamental to structural and functional brain organization. This insight not only has implications for how we should collect and analyse data, it may also help us to understand how the different levels of skewed distributions - from synapses to cognition - are related to each other.
Figures



References
-
- Barabasi AL, Albert R. Emergence of scaling in random networks. Science. 1999;286:509–512. - PubMed
-
- Sporns O. Networks of the Brain. MIT Press; 2010.
-
- Limpert E, Stahel WA, Abbt M. Lognormal distributions across the sciences: keys and clues. Bioscience. 2001;51:341–352.
-
- Weber H. Annotationes Anatomicae et Physiologicae. Koehler; German: 1834. De Pulsa Resorptione Auditu et Tactu.
-
- Fechner GT. Elemente der Psychophysik. Breitkopf und Härtel; German: 1860.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

