Cell segregation in the vertebrate hindbrain relies on actomyosin cables located at the interhombomeric boundaries
- PMID: 24569501
- PMCID: PMC4000087
- DOI: 10.1002/embj.201386003
Cell segregation in the vertebrate hindbrain relies on actomyosin cables located at the interhombomeric boundaries
Abstract
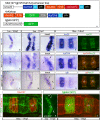
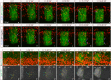
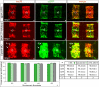
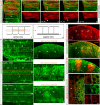
Segregating cells into compartments during embryonic development is essential for growth and pattern formation. Physical mechanisms shaping compartment boundaries were recently explored in Drosophila, where actomyosin-based barriers were revealed to be important for keeping cells apart. In vertebrates, interhombomeric boundaries are straight interfaces, which often serve as signaling centers that pattern the surrounding tissue. Here, we demonstrate that in the hindbrain of zebrafish embryos cell sorting sharpens the molecular boundaries and, once borders are straight, actomyosin barriers are key to keeping rhombomeric cells segregated. Actomyosin cytoskeletal components are enriched at interhombomeric boundaries, forming cable-like structures in the apical side of the neuroepithelial cells by the time morphological boundaries are visible. When myosin II function is inhibited, cable structures do not form, leading to rhombomeric cell mixing. Downregulation of EphA4a compromises actomyosin cables and cells with different rhombomeric identity intermingle, and the phenotype is rescued enhancing myosin II activity. Moreover, enrichment of actomyosin structures is obtained when EphA4 is ectopically expressed in even-numbered rhombomeres. These findings suggest that mechanical barriers act downstream of EphA/ephrin signaling to segregate cells from different rhombomeres.
Figures



Similar articles
-
Cell segregation in the vertebrate hindbrain: a matter of boundaries.Cell Mol Life Sci. 2015 Oct;72(19):3721-30. doi: 10.1007/s00018-015-1953-8. Epub 2015 Jun 19. Cell Mol Life Sci. 2015. PMID: 26089248 Free PMC article. Review.
-
An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos.Nat Cell Biol. 2010 Jan;12(1):60-9. doi: 10.1038/ncb2005. Epub 2009 Dec 6. Nat Cell Biol. 2010. PMID: 19966783 Free PMC article.
-
EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish.Curr Biol. 2005 Mar 29;15(6):536-42. doi: 10.1016/j.cub.2005.02.019. Curr Biol. 2005. PMID: 15797022
-
Evolutionary emergence of the rac3b/rfng/sgca regulatory cluster refined mechanisms for hindbrain boundaries formation.Proc Natl Acad Sci U S A. 2018 Apr 17;115(16):E3731-E3740. doi: 10.1073/pnas.1719885115. Epub 2018 Apr 2. Proc Natl Acad Sci U S A. 2018. PMID: 29610331 Free PMC article.
-
The multiple functions of hindbrain boundary cells: Tinkering boundaries?Semin Cell Dev Biol. 2020 Nov;107:179-189. doi: 10.1016/j.semcdb.2020.05.002. Epub 2020 May 22. Semin Cell Dev Biol. 2020. PMID: 32448645 Review.
Cited by
-
An LRR Receptor-Teneurin System Directs Planar Polarity at Compartment Boundaries.Dev Cell. 2019 Oct 21;51(2):208-221.e6. doi: 10.1016/j.devcel.2019.08.003. Epub 2019 Sep 5. Dev Cell. 2019. PMID: 31495696 Free PMC article.
-
Adhesion-Based Self-Organization in Tissue Patterning.Annu Rev Cell Dev Biol. 2022 Oct 6;38:349-374. doi: 10.1146/annurev-cellbio-120420-100215. Epub 2022 May 13. Annu Rev Cell Dev Biol. 2022. PMID: 35562853 Free PMC article. Review.
-
Regulation of cell differentiation by Eph receptor and ephrin signaling.Cell Adh Migr. 2014;8(4):339-48. doi: 10.4161/19336918.2014.970007. Cell Adh Migr. 2014. PMID: 25482623 Free PMC article. Review.
-
Sorting at embryonic boundaries requires high heterotypic interfacial tension.Nat Commun. 2017 Jul 31;8(1):157. doi: 10.1038/s41467-017-00146-x. Nat Commun. 2017. PMID: 28761157 Free PMC article.
-
Morphogenesis of extra-embryonic tissues directs the remodelling of the mouse embryo at implantation.Nat Commun. 2019 Aug 7;10(1):3557. doi: 10.1038/s41467-019-11482-5. Nat Commun. 2019. PMID: 31391456 Free PMC article.
References
-
- Aliee M, Röper JC, Landsberg KP, Pentzold C, Widmann TJ, Jülicher F, Dahmann C. Physical mechanisms shaping the Drosophila dorsoventral compartment boundary. Curr Biol. 2012;22:967–976. - PubMed
-
- Becam I, Rafel N, Hong X, Cohen SM, Milán M. Notch-mediated repression of bantam miRNA contributes to boundary formation in the Drosophila wing. Development. 2011;138:3781–3789. - PubMed
-
- Behrndt M, Salbreux G, Campinho P, Hauschild R, Oswald F, Roensch J, Grill SW, Heisenberg CP. Forces driving epithelial spreading in zebrafish gastrulation. Science. 2012;338:257–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

