Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer
- PMID: 24569566
- PMCID: PMC3935187
- DOI: 10.1038/srep04202
Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer
Abstract
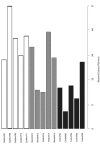
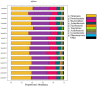
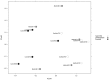
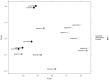
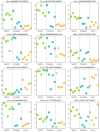
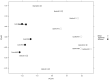
We aimed to characterize microbiota of the gastric mucosa as it progress to intestinal type of cancer. Study included five patients each of non-atrophic gastritis (NAG), intestinal metaplasia (IM) and intestinal-type gastric cancer (GC). Gastric tissue was obtained and DNA extracted for microbiota analyses using the microarray G3 PhyloChip. Bacterial diversity ranged from 8 to 57, and steadily decreased from NAG to IM to GC (p = 0.004). A significant microbiota difference was observed between NAG and GC based on Unifrac-presence/absence and weighted-Unifrac-abundance metrics of 283 taxa (p < 0.05). HC-AN analyses based on presence/absence of 238 taxa revealed that GC and NAG grouped apart, whereas IM overlapped with both. An ordinated analyses based on weighted-Unifrac distance given abundance of 44 taxa showing significance across categories revealed significant microbiota separation between NAG and GC. This study is the first to show a gradual shift in gastric microbiota profile from NAG to IM to GC.
Figures

References
-
- Bjorkholm B., Falk P., Engstrand L. & Nyren O. Helicobacter pylori: resurrection of the cancer link. J Intern Med 253, 102–119, 1119 (2003). - PubMed
-
- Allison C. C. & Ferrero R. L. Role of virulence factors and host cell signaling in the recognition of Helicobacter pylori and the generation of immune responses. Future Microbiol 5, 1233–1255, 10.2217/fmb.10.84 (2010). - PubMed
-
- Tsugane S. & Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer 10, 75–83, 10.1007/s10120-007-0420-0 (2007). - PubMed
-
- Correa P. & Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology 133, 659–672, 10.1053/j.gastro.2007.06.026 (2007). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

