Tuning the electrical properties of the heart by differential trafficking of KATP ion channel complexes
- PMID: 24569881
- PMCID: PMC4004980
- DOI: 10.1242/jcs.141440
Tuning the electrical properties of the heart by differential trafficking of KATP ion channel complexes
Abstract
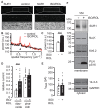
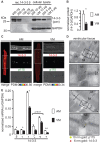
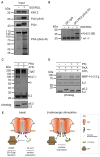
The copy number of membrane proteins at the cell surface is tightly regulated. Many ion channels and receptors present retrieval motifs to COPI vesicle coats and are retained in the early secretory pathway. In some cases, the interaction with COPI is prevented by binding to 14-3-3 proteins. However, the functional significance of this antagonism between COPI and 14-3-3 in terminally differentiated cells is unknown. Here, we show that ATP-sensitive K(+) (KATP) channels, which are composed of Kir6.2 and SUR1 subunits, are stalled in the Golgi complex of ventricular, but not atrial, cardiomyocytes. Upon sustained β-adrenergic stimulation, which leads to activation of protein kinase A (PKA), SUR1-containing channels reach the plasma membrane of ventricular cells. We show that PKA-dependent phosphorylation of the C-terminus of Kir6.2 decreases binding to COPI and, thereby, silences the arginine-based retrieval signal. Thus, activation of the sympathetic nervous system releases this population of KATP channels from storage in the Golgi and, hence, might facilitate the adaptive response to metabolic challenges.
Keywords: 14-3-3; ATP-sensitive K+ channels; Arg-based retrieval signal; COPI; Cardiomyocyte; Coatomer; KATP; PKA; Protein kinase A; Trafficking.
Figures





Similar articles
-
Role of the C-terminus of SUR in the differential regulation of β-cell and cardiac KATP channels by MgADP and metabolism.J Physiol. 2018 Dec;596(24):6205-6217. doi: 10.1113/JP276708. Epub 2018 Oct 14. J Physiol. 2018. PMID: 30179258 Free PMC article.
-
ATP binding without hydrolysis switches sulfonylurea receptor 1 (SUR1) to outward-facing conformations that activate KATP channels.J Biol Chem. 2019 Mar 8;294(10):3707-3719. doi: 10.1074/jbc.RA118.005236. Epub 2018 Dec 26. J Biol Chem. 2019. PMID: 30587573 Free PMC article.
-
Sulfonylurea receptors type 1 and 2A randomly assemble to form heteromeric KATP channels of mixed subunit composition.J Gen Physiol. 2008 Jan;131(1):43-58. doi: 10.1085/jgp.200709894. Epub 2007 Dec 17. J Gen Physiol. 2008. PMID: 18079561 Free PMC article.
-
Human K(ATP) channelopathies: diseases of metabolic homeostasis.Pflugers Arch. 2010 Jul;460(2):295-306. doi: 10.1007/s00424-009-0771-y. Epub 2009 Dec 24. Pflugers Arch. 2010. PMID: 20033705 Free PMC article. Review.
-
Molecular biology of adenosine triphosphate-sensitive potassium channels.Endocr Rev. 1999 Apr;20(2):101-35. doi: 10.1210/edrv.20.2.0361. Endocr Rev. 1999. PMID: 10204114 Review.
Cited by
-
Phosphorylation-dependent changes in nucleotide binding, conformation, and dynamics of the first nucleotide binding domain (NBD1) of the sulfonylurea receptor 2B (SUR2B).J Biol Chem. 2015 Sep 11;290(37):22699-714. doi: 10.1074/jbc.M114.636233. Epub 2015 Jul 21. J Biol Chem. 2015. PMID: 26198630 Free PMC article.
-
Dual RXR motifs regulate nerve growth factor-mediated intracellular retention of the delta opioid receptor.Mol Biol Cell. 2019 Mar 1;30(5):680-690. doi: 10.1091/mbc.E18-05-0292. Epub 2019 Jan 2. Mol Biol Cell. 2019. PMID: 30601694 Free PMC article.
-
Analysis of tubular membrane networks in cardiac myocytes from atria and ventricles.J Vis Exp. 2014 Oct 15;(92):e51823. doi: 10.3791/51823. J Vis Exp. 2014. PMID: 25350293 Free PMC article.
-
Genetic variations involved in sudden cardiac death and their associations and interactions.Heart Fail Rev. 2016 Jul;21(4):401-14. doi: 10.1007/s10741-016-9563-6. Heart Fail Rev. 2016. PMID: 27241195 Review.
-
Axial Tubule Junctions Activate Atrial Ca2+ Release Across Species.Front Physiol. 2018 Oct 8;9:1227. doi: 10.3389/fphys.2018.01227. eCollection 2018. Front Physiol. 2018. PMID: 30349482 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

