Discovery of PI-1840, a novel noncovalent and rapidly reversible proteasome inhibitor with anti-tumor activity
- PMID: 24570003
- PMCID: PMC4002098
- DOI: 10.1074/jbc.M113.533950
Discovery of PI-1840, a novel noncovalent and rapidly reversible proteasome inhibitor with anti-tumor activity
Abstract
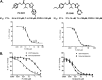
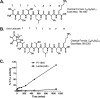
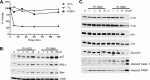
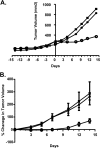
The proteasome inhibitor bortezomib is effective in hematologic malignancies such as multiple myeloma but has little activity against solid tumors, acts covalently, and is associated with undesired side effects. Therefore, noncovalent inhibitors that are less toxic and more effective against solid tumors are desirable. Structure activity relationship studies led to the discovery of PI-1840, a potent and selective inhibitor for chymotrypsin-like (CT-L) (IC50 value = 27 ± 0.14 nm) over trypsin-like and peptidylglutamyl peptide hydrolyzing (IC50 values >100 μm) activities of the proteasome. Furthermore, PI-1840 is over 100-fold more selective for the constitutive proteasome over the immunoproteasome. Mass spectrometry and dialysis studies demonstrate that PI-1840 is a noncovalent and rapidly reversible CT-L inhibitor. In intact cancer cells, PI-1840 inhibits CT-L activity, induces the accumulation of proteasome substrates p27, Bax, and IκB-α, inhibits survival pathways and viability, and induces apoptosis. Furthermore, PI-1840 sensitizes human cancer cells to the mdm2/p53 disruptor, nutlin, and to the pan-Bcl-2 antagonist BH3-M6. Finally, in vivo, PI-1840 but not bortezomib suppresses the growth in nude mice of human breast tumor xenografts. These results warrant further evaluation of a noncovalent and rapidly reversible proteasome inhibitor as potential anticancer agents against solid tumors.
Keywords: Anticancer Drug; Apoptosis; Bcl-2 Family Proteins; CT-L; Cancer Therapy; Cell Proliferation; Noncovalent; PI-1840; Proteasome; p53.
Figures

References
-
- Pagano M., Benmaamar R. (2003) When protein destruction runs amok, malignancy is on the loose. Cancer Cell 4, 251–256 - PubMed
-
- Schwartz A. L., Ciechanover A. (1999) The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu. Rev. Med. 50, 57–74 - PubMed
-
- Adams J. (2004) The development of proteasome inhibitors as anticancer drugs. Cancer Cell 5, 417–421 - PubMed
-
- Adams J. (2003) The proteasome: structure, function, and role in the cell. Cancer Treat. Rev. 29, 3–9 - PubMed
-
- Ciechanover A. (1994) The ubiquitin-proteasome proteolytic pathway. Cell 79, 13–21 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

